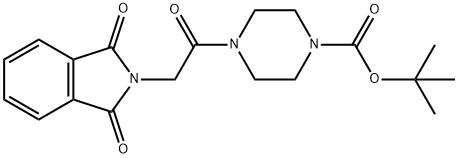
tert-butyl 4-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetyl]piperazine-1-carboxylate synthesis
- Product Name:tert-butyl 4-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetyl]piperazine-1-carboxylate
- CAS Number:1010925-33-3
- Molecular formula:C19H23N3O5
- Molecular Weight:373.4
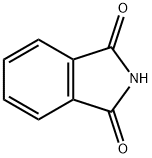
85-41-6
470 suppliers
$9.00/1g
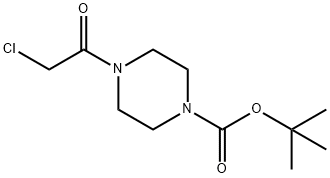
190001-40-2
43 suppliers
$48.00/250mg
![tert-butyl 4-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetyl]piperazine-1-carboxylate](/CAS/20180703/GIF/1010925-33-3.gif)
1010925-33-3
4 suppliers
inquiry
Yield:1010925-33-3 67%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;N-ethyl-N,N-diisopropylamine in dichloromethane at 40; for 24 h;
Steps:
Phthalimide (33 mg, 0.23 mmol) was treated with fe/f-butyl 4-(2-chloroacetyl)piperazine-1 -carboxylate (80.5 mg, 0.32 mmol), tetrabutylammonium iodide (TBAI, 84 mg, 2.3 mmol), diisopropylethylamine (DIEA, 0.20 mg, 1 .14 mmol) in anhydrous CH2CI2 (10 ml_) for 24 h at 40 °C. The mixture was partitioned between CH2CI2 and water. The organic phase was separated, dried over MgS04, filtered, concentrated, and purified by flash chromatography on a silica gel column with elution of EtOAc/hexane (1 :1 ) to give the title compound (57.1 mg, 67% yield). white solid; TLC (EtOAc/hexane = 1 : 1 ) Rf = 0.2; 1 H NMR (400 MHz, CDCI3) δ 7.85-7.87 (2 H, m), 7.70-7.72 (2 H, m), 4.49 (2 H, s), 3.51-3.57 (6 H, m), 3.44 (2 H, d, J = 5.2 Hz), 1 .46 (9 H, d, J = 4.8 Hz).
References:
WO2012/72019,2012,A1 Location in patent:Page/Page column 71

24424-99-5
821 suppliers
$13.50/25G
![tert-butyl 4-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetyl]piperazine-1-carboxylate](/CAS/20180703/GIF/1010925-33-3.gif)
1010925-33-3
4 suppliers
inquiry

57260-71-6
740 suppliers
$5.00/5g
![tert-butyl 4-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetyl]piperazine-1-carboxylate](/CAS/20180703/GIF/1010925-33-3.gif)
1010925-33-3
4 suppliers
inquiry