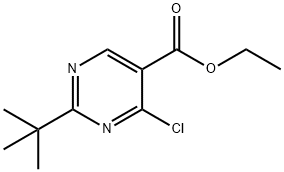
ethyl 2-tert-butyl-4-chloropyrimidine-5-carboxylate synthesis
- Product Name:ethyl 2-tert-butyl-4-chloropyrimidine-5-carboxylate
- CAS Number:1011464-42-8
- Molecular formula:C11H15ClN2O2
- Molecular Weight:242.7
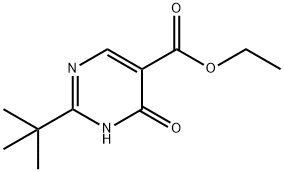
827014-34-6
6 suppliers
inquiry

1011464-42-8
15 suppliers
inquiry
Yield:1011464-42-8 95%
Reaction Conditions:
with triethylamine;trichlorophosphate at 40; for 1 h;
Steps:
30.2 Step 2: ethyl 2-(tert-butyl)-4-chloropyrimidine-5-carboxylate
At 0° C., Et3N (1.1 mL) was added to POCl3 (32 mL), followed by the addition of ethyl 2-tert-butyl-4-hydroxypyrimidine-5-carboxylate (3.70 g, 16.5 mmol). The mixture was stirred at 40° C. for 1 h and the excess POCl3 was removed under vacuum. The residue was carefully poured into ice (400 mL) and was extracted with CH2Cl2. The extract was washed with NaHCO3 and water, dried over Na2SO4, filtered and concentrated. The crude material was subjected to silica gel column chromatography (10-90% CH2Cl2/hexanes) to afford ethyl 2-(tert-butyl)-4-chloropyrimidine-5-carboxylate (3.8 g, 95%) as a liquid. (M+1)+=243.4.
References:
US2016/95858,2016,A1 Location in patent:Paragraph 2903; 2904

827014-34-6
6 suppliers
inquiry

1011464-42-8
15 suppliers
inquiry
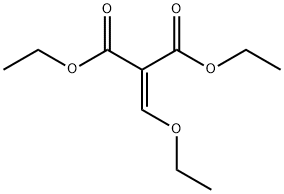
87-13-8
426 suppliers
$5.00/5g

1011464-42-8
15 suppliers
inquiry
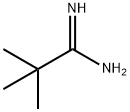
59950-56-0
20 suppliers
$105.00/1g

1011464-42-8
15 suppliers
inquiry
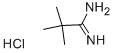
18202-73-8
124 suppliers
$7.00/250mg

1011464-42-8
15 suppliers
inquiry