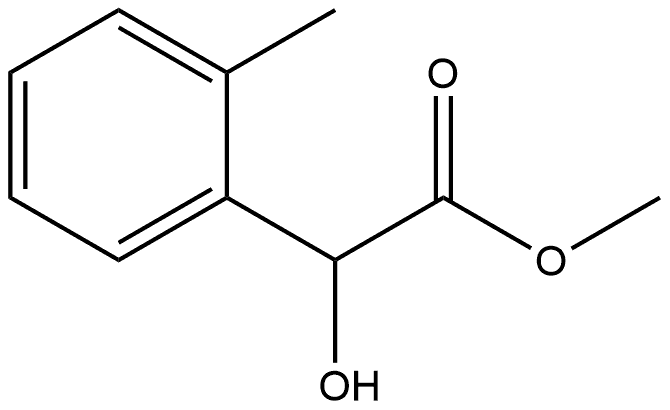
Benzeneacetic acid, α-hydroxy-2-methyl-, methyl ester, (-)- synthesis
- Product Name:Benzeneacetic acid, α-hydroxy-2-methyl-, methyl ester, (-)-
- CAS Number:1011722-47-6
- Molecular formula:C10H12O3
- Molecular Weight:180.2
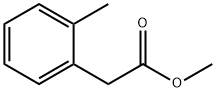
40851-62-5
60 suppliers
$9.00/1g

1011722-47-6
0 suppliers
inquiry
Yield:1011722-47-6 50 %
Reaction Conditions:
Stage #1: methyl o-tolylacetatewith 4-toluenesulfonyl azide;1,8-diazabicyclo[5.4.0]undec-7-ene in acetonitrile at 0 - 20;
Stage #2: with water in N,N-dimethyl-formamide at 20; under 760.051 Torr;Inert atmosphere;Irradiation;
Steps:
General procedure for the O-H insertion reactions of water (GP-1)
General procedure: A solution of diazo alkane (0.1 mmol, 1.0 eq.) and ultrapure water (0.5 mmol, 5.0 eq.) in 1.0 ml dryDMF to the reaction tube at room temperature. Fill the reaction system with 1 atm nitrogen, Thereaction mixture was stirred at room temperature for 8 hours under the irradiation of 1W blue LED.The reaction system was quenched with 20ml water, extracted twice with 10ml EtOAc, thecombined organic layer was washed with 10ml saturated sodium chloride solution, the organic layerwas collected and dried over anhydrous magnesium sulfate for 0.5h, the solvent was evaporatedunder reduced pressure, and the residue was purified by silica gel column chromatography(petroleum ether/ethyl acetate).
References:
Bai, Jinrui;Qi, Dan;Song, Zhuoheng;Li, Bin;Guo, Lin;Yang, Chao;Xia, Wujiong [Synlett,2022,vol. 33,# 20,p. 2048 - 2052] Location in patent:supporting information