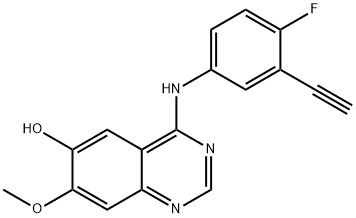
4-((3-ethynyl-4-fluorophenyl)amino)-7-methoxyquinazolin-6-ol synthesis
- Product Name:4-((3-ethynyl-4-fluorophenyl)amino)-7-methoxyquinazolin-6-ol
- CAS Number:1012057-64-5
- Molecular formula:C17H12FN3O2
- Molecular Weight:309.29

1012057-63-4
4 suppliers
inquiry

1012057-64-5
4 suppliers
inquiry
Yield:1012057-64-5 100%
Reaction Conditions:
Stage #1: 4-((3-ethynyl-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl acetatewith lithium hydroxide;water in methanol at 20; for 0.5 h;
Stage #2: with acetic acid in methanol;water;
Steps:
31.31f
A mixture of compound 0606 (260 mg, 0.74 mmol), LiOH H2O (250 mg, 5.8 mmol) in methanol (25 ml) and H2O (25 ml) was stirred at room temperature for 0.5 hour. The mixture was neutralized by addition of dilution acetic acid. The precipitate was isolated and dried to give the title compound 0607 (234 mg, 100%) as a grey solid: LCMS: 310[M+l]+.
References:
WO2008/33747,2008,A2 Location in patent:Page/Page column 140

1072-85-1
452 suppliers
$5.00/10g

1012057-64-5
4 suppliers
inquiry
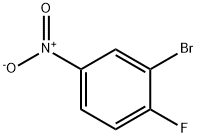
701-45-1
273 suppliers
$9.00/1g

1012057-64-5
4 suppliers
inquiry
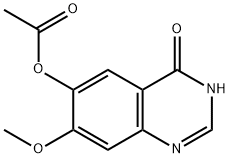
179688-53-0
275 suppliers
$6.00/1g

1012057-64-5
4 suppliers
inquiry
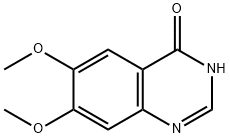
13794-72-4
285 suppliers
$8.00/1g

1012057-64-5
4 suppliers
inquiry