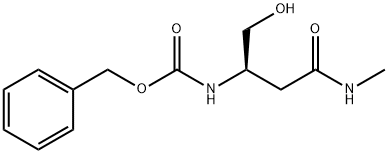
(R)-benzyl 1-hydroxy-4-(methylamino)-4-oxobutan-2-ylcarbamate synthesis
- Product Name:(R)-benzyl 1-hydroxy-4-(methylamino)-4-oxobutan-2-ylcarbamate
- CAS Number:1012059-95-8
- Molecular formula:C13H18N2O4
- Molecular Weight:266.29
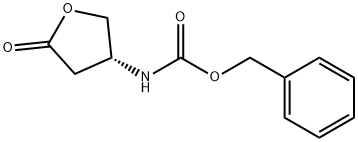
118399-28-3
138 suppliers
$6.00/100mg

74-89-5
0 suppliers
$13.44/25ML

1012059-95-8
10 suppliers
inquiry
Yield:1012059-95-8 88%
Reaction Conditions:
in ethanol; for 0.25 h;
Steps:
1.b
0101 (5.04 g, 21.4 mmol) was added into a solution of methanamine (31.06g, 1 mol) in ethanol (100 ml) and stirred for 15 m, during this period 0101 was dissolved gradually and then new solid appeared. The solvent was evaporated under reduced pressure to obtain 0102 (5.016 g, 88%) as a white solid which was used in the next step reaction without further purification. LCMS: 267 [M+l]+; 1H NMR (DMSO-J6): δ 2.18 (dd, IH, J1 = 8.4 Hz5 J2 = 14.1 Hz), 2.31 (dd, IH, J1 = 6.3 Hz5 J2 = 14.4 Hz), 2.54 (d, 3H, J= 5.1 Hz), 3.33 (m, IH), 3.82 (m, IH), 4.703 (m, IH), 5.00 (s, 2H), 6.98 (d, IH, J= 8.4 Hz), 7.35 (m, 5H), 7.68 (m, IH).
References:
WO2009/36051,2009,A1 Location in patent:Page/Page column 48