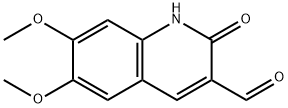
6,7-DiMethoxy-2-oxo-1,2-dihydro-quinoline-3-carbaldehyde synthesis
- Product Name:6,7-DiMethoxy-2-oxo-1,2-dihydro-quinoline-3-carbaldehyde
- CAS Number:101382-56-3
- Molecular formula:C12H11NO4
- Molecular Weight:233.22

68236-23-7
46 suppliers
$39.00/1g

101382-56-3
16 suppliers
$99.00/1g
Yield:-
Reaction Conditions:
with acetic acid in water;Reflux;
Steps:
4.1.1. Synthesis of 2-oxoquinoline-3-carbaldehydes (1a-c) and 3-(hydroxymethyl)quinolinones (IVa-c) [34]
General procedure: These compounds were prepared by following the Meth-Cohnmethod. N,N-dimethylformamide (9.6 mL, 0.125 mol) was cooled to0 °C and phosphoryl chloride (32.2 mL, 0.35 mol) was added dropwisewith stirring. To this solution, the corresponding acetanilides IIa-c(0.05 mol) was added and the temperature of the reaction mixture wasraised to 80 °C during 8-19 h. The cooled reaction mixture was pouredinto ice-water (300 mL) and stirred for 10 min at 0-10 °C. The precipitateformed corresponding to 2-chloroquinoline-3-carbaldehydesIIIa-c was filtered off, washed with water (100 mL), dried, and recrystallizedfrom acetonitrile. A suspension of these aldehydes III(1 mmol) in 70% aqueous acetic acid (50 mL) was heated under refluxfor 24-72 h. Completion of the reaction was checked by TLC. Aftercooling the solid formed was filtered, washed well with water, driedand purified by recrystallization from DMF to obtain 1a-c. To a stirred solution of 2-oxo-1,2-dihydroquinoline-3-carbaldehydes 1a-c (1 mmol)in 15 mL methanol sodium borohydride (3 mmol) was slowly added atroom temperature. The progress reaction was monitored by TLC (20:1DCM:MeOH). After completion of the reaction (18 h), the solvent wasremoved under reduced pressure. To the residue formed, ice cold waterwas added and the obtained solid was filtered off and recrystallizedfrom MeOH to obtain 1d-f. To a stirred solution of 1d-f (1 mmol) inDCM (10 mL), a solution of SOCl2 (2 mL) in DCM (5 mL) was addeddropwise. After the addition was complete, four drops of DMF wasadded and the mixture was stirred for 2 h at reflux. The reaction progresswas monitored by the TLC (20:1 DCM:MeOH). After the solventwas removed under reduced pressure the compound 3-(chloromethyl)-quinolin-2(1H)-ones 1g-i were obtained as a syrup and further usedwithout purification.
References:
Abonia, Rodrigo;Andújar, Sebastián;Cobo, Justo;Enriz, Ricardo D.;Gutiérrez, Lucas;Insuasty, Daniel;Lima, Santiago;Marchal, Antonio;Nogueras, Manuel;Spiegel, Sarah;Vettorazzi, Marcela [Bioorganic Chemistry,2019]
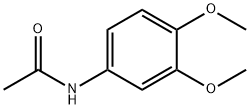
881-70-9
31 suppliers
$105.00/500mg

101382-56-3
16 suppliers
$99.00/1g
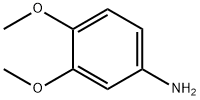
6315-89-5
314 suppliers
$7.00/10g

101382-56-3
16 suppliers
$99.00/1g