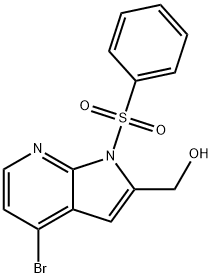
1H-Pyrrolo[2,3-b]pyridine-2-Methanol, 4-broMo-1-(phenylsulfonyl)- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine-2-Methanol, 4-broMo-1-(phenylsulfonyl)-
- CAS Number:1014613-32-1
- Molecular formula:C14H11BrN2O3S
- Molecular Weight:367.22

50-00-0
846 suppliers
$10.00/25g
![1H-Pyrrolo[2,3-b]pyridine-2-Methanol, 4-broMo-1-(phenylsulfonyl)-](/CAS/GIF/1014613-32-1.gif)
1014613-32-1
1 suppliers
inquiry
Yield:1014613-32-1 23%
Reaction Conditions:
Stage #1: 1-(benzenesulfonyl)-4-bromo-2-methyl-1H-pyrrolo[2,3-b]pyridinewith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -40; for 0.5 h;
Stage #2: formaldehyd in tetrahydrofuran;n-heptane;ethylbenzene at -60; for 3 h;
Steps:
To a solution of 4-bromo-2-methyl-1-(phenylsulfonyl)-1/-/-pyrrolo[2,3-6]pyridine (10.Og, 29.7mmol) in dry THF (150ml) at -4O0C under nitrogen was added 2M LDA in heptane/THF/ethylbenzene (30.0ml, βO.Ommol) drop wise. The reaction was stirred -4O0C for 30 min then cooled to -6O0C. Paraformaldehyde (7g, 23.3mmol) was added in one portion. After 3 hrs the reaction was cooled to -6O0C and saturated ammonium chloride solution (100ml) was added to pH5-6. It was allowed to warm to room temperature and extracted with DCM (2x100ml). The combined extracts were washed with 2M hydrochloric acid, brine, dried (MgSO4) and evaporated to give a brown oil (10g). Purification was by silica SPE cartridge (5Og) eluting with DCM to 90% DCM / EtOAc to give the title compound as a yellow foam (2.5g, 23%). MH+369, rt= 1.03 min
References:
WO2008/34860,2008,A1 Location in patent:Page/Page column 128
![4-BROMO-1-(PHENYLSULFONYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/889939-25-7.gif)
889939-25-7
93 suppliers
$38.00/250mg
![1H-Pyrrolo[2,3-b]pyridine-2-Methanol, 4-broMo-1-(phenylsulfonyl)-](/CAS/GIF/1014613-32-1.gif)
1014613-32-1
1 suppliers
inquiry