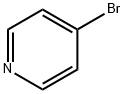
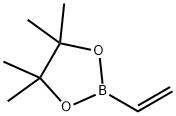
Pyridine, 4-[(1E)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl]- synthesis
- Product Name:Pyridine, 4-[(1E)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)ethenyl]-
- CAS Number:1015243-13-6
- Molecular formula:C13H18BNO2
- Molecular Weight:231.1
Yield:1015243-13-6 70%
Reaction Conditions:
with tris(1,5-diphenylpenta-1,4-dien-3-one)dipalladium;N-ethyl-N,N-diisopropylamine;tri-tert-butylphosphonium hydrogen tetrafluoroborate in toluene at 20 - 85; for 15 h;Inert atmosphere;Time;
Steps:
242.1; 278.1 Step 1 : (E)-4-( 2-( 4, 4, 5, 5-Tetramethyl-l, 3, 2-dioxaborolan-2-yl)vinyl)pyridine
A mixture of 4-bromopyridine (3 g, 18.99 mmol), 4, 4,5, 5-tetramethyl-2 -vinyl-1, 3,2- dioxaborolane (3.51 g, 22.79 mmol, 3.86 mL), tri-/c/7-butylphosphanium tetrafluoroborate (550.89 mg, 1.90 mmol), Pd2(dba)3 (869.37 mg, 949.39 umol) and DIEA (4.91 g, 37.98 mmol, 6.61 mL) in toluene (15 mL) was degassed and purged with N2 (3x) at 20 °C. The mixture was then stirred at 85 °C for 15 hrs under an N2 atmosphere. The mixture was concentrated to give a residue that was purified by silica gel column chromatography using a 0-26% EtOAc/petroleum ether gradient eluent to afford the title compound (3.1 g, 70%) as a yellow solid. 'H NMR (400 MHz, CDCh) 8 8.61 (br d, J=5.01 Hz, 2 H), 7.34 (d, J=6.36 Hz, 2 H), 7.28 (d, J=7.21 Hz, 1 H), 6.39 (d, J=18.34 Hz, 1 H), 1.33 (s, 12 H). MS-ESI (m/z) calc’d for C13H19BNO2 [M+H]+: 232.1. Found 232.3.
References:
WO2022/155419,2022,A1 Location in patent:Page/Page column 437-438; 504-505