
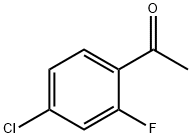
Benzenemethanol, 4-chloro-2-fluoro-α-methyl-, (αR)- synthesis
- Product Name:Benzenemethanol, 4-chloro-2-fluoro-α-methyl-, (αR)-
- CAS Number:1016646-16-4
- Molecular formula:C8H8ClFO
- Molecular Weight:174.6
175711-83-8
154 suppliers
$8.00/1g

1016646-16-4
1 suppliers
inquiry
Yield:1016646-16-4 18.8 g
Reaction Conditions:
Stage #1: 4’-chloro-2’-fluoroacetophenonewith B-chlorodiisopinocampheylborane in tetrahydrofuran;hexane at -78 - -25; for 7 h;Inert atmosphere;
Stage #2: with 2,2'-iminobis[ethanol] in tetrahydrofuran;hexane at 20; for 72 h;
Steps:
49.1 Step 1: (R)-1-(4-Chloro-2-fluorophenyl)ethan-1-ol
To a solution of 1-(4-chloro-2-fluorophenyl)ethan-1-one (10 g, 58 mmol) in THF (100 mL) at -78° C. under a nitrogen atmosphere was added (+)-DIP-Cl (50 to 60% wt in hexanes, 9.2 mL, 64 mmol), slowly.
The resulting mixture was slowly warmed to -25° C. and stirred at this temperature for 2 h.
1-(4-Chloro-2-fluorophenyl)ethan-1-one was detected by LCMS and HPLC, and the mixture was cooled back to -78° C.
Additional (+)-DIP-Cl (50 to 65% wt in hexanes, 5.4 mL, 38 mmol) was added to the mixture at -78° C.
The resulting mixture was slowly warmed to -25° C. and stirred at this temperature for 5 h.
Diethanolamine (18 mL, 191 mmol) was added to the reaction mixture, which was then stirred at room temperature for 3 d.
The reaction was filtered, and the filtrate was concentrated and purified by silica gel chromatography (0 to 30% ethyl acetate in hexanes) to afford 18.8 g impure (R)-1-(chloro-2-fluorophenyl)ethan-1-ol.
References:
US2018/72743,2018,A1 Location in patent:Paragraph 1489-1490