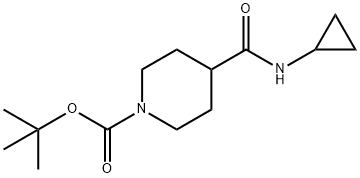
tert-Butyl 4-(cyclopropylcarbaMoyl)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(cyclopropylcarbaMoyl)piperidine-1-carboxylate
- CAS Number:1016743-04-6
- Molecular formula:C14H24N2O3
- Molecular Weight:268.35
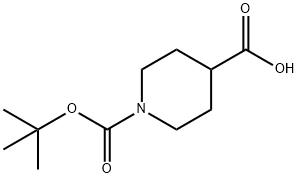
84358-13-4
391 suppliers
$5.00/5g
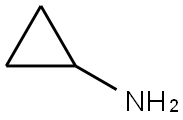
765-30-0
487 suppliers
$10.00/25g

1016743-04-6
11 suppliers
$175.00/500mg
Yield:1016743-04-6 64%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;HATU in N,N-dimethyl-formamide at 20 - 27; for 3 h;
Steps:
1-(tert-Butoxycarbonyl) piperidine-4-carboxylic acid (2.0 g, 8.7 mmol) and cyclopropylamine (0.6 mL, 8.7 mL) were dissolved in DMF (45 mL). HATU (3.3 g, 8.7 mmol) was added at room temperature followed by DIPEA (3.1 mL, 17 mmol). The reaction mixture was stirred at roomtemperature for 3h. The reaction mixture was diluted with cold water (250 mL) and extracted with EtOAc (3 x 100 mL). The combined organic layers were dried (Na2SO4) and concentrated to give the crude product, which was purified by column chromatography (Normal phase, Neutral silica gel, 60-120 mesh, 0 to 35 % EtOAC in hexanes) to give tert-butyl 4- (cyclopropylcarbamoyl)piperidine-1-carboxylate (1.5 g, 64%) as a solid.LCMS (Method F): mlz 269 (M+H) (ES), at 1.80 mm, weakly UV active.
References:
WO2015/118342,2015,A1 Location in patent:Page/Page column 84
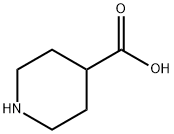
498-94-2
492 suppliers
$5.00/10g

1016743-04-6
11 suppliers
$175.00/500mg

24424-99-5
824 suppliers
$13.50/25G

1016743-04-6
11 suppliers
$175.00/500mg