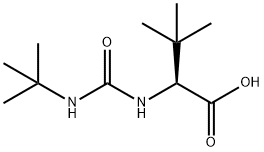
N-[[(1,1-DiMethylethyl)aMino]carbonyl]-3-Methyl-L-valine synthesis
- Product Name:N-[[(1,1-DiMethylethyl)aMino]carbonyl]-3-Methyl-L-valine
- CAS Number:101968-85-8
- Molecular formula:C11H22N2O3
- Molecular Weight:230.3

20859-02-3
604 suppliers
$9.00/5G

1609-86-5
206 suppliers
$33.39/5g
![N-[[(1,1-DiMethylethyl)aMino]carbonyl]-3-Methyl-L-valine](/CAS/GIF/101968-85-8.gif)
101968-85-8
108 suppliers
$11.00/1g
Yield:101968-85-8 98%
Reaction Conditions:
Stage #1: L-tert-Leucinewith sodium hydroxide in water; pH=11.5;
Stage #2: Tert-butyl isocyanate in water; pH=9.3;Cooling with ice;
Steps:
2
The pH of a stirred aqueous solution (1150 g) containing L-tert-leucine (100 g, 762 mmol) was adjusted to 11.5 using a 48% by weight aqueous sodium hydroxide solution. Thereafter, tert-butyl isocyanate (75.5 g, 762 mmol) was slowly added thereto in an ice bath. The reaction was completed in 6 hours after the addition. The reaction mixture was analyzed with HPLC; as a result, it was found that 172 g of an N-tert-butylcarbamoyl-L-tert-leucine was generated (yield: 98%), and 0.02% of a dipeptide-like compound and 0.07% of N,N'-di(tert-butyl)urea were generated. The amount of the enantiomer was below measurable limits, i.e., 0.01%. The pH of the reaction mixture after the reaction was 9.3.
References:
EP2221294,2010,A1 Location in patent:Page/Page column 6-7

20859-02-3
604 suppliers
$9.00/5G
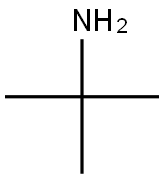
75-64-9
424 suppliers
$10.00/10g
530-62-1
930 suppliers
$6.00/25g
![N-[[(1,1-DiMethylethyl)aMino]carbonyl]-3-Methyl-L-valine](/CAS/GIF/101968-85-8.gif)
101968-85-8
108 suppliers
$11.00/1g