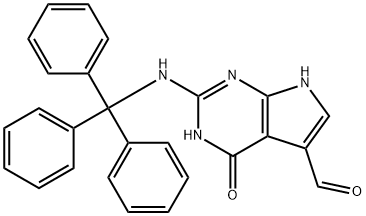
4,7-Dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde synthesis
- Product Name:4,7-Dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde
- CAS Number:1019853-65-6
- Molecular formula:C26H20N4O2
- Molecular Weight:420.46
![4,7-Dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile](/CAS/20180527/GIF/1019853-64-5.gif)
1019853-64-5
7 suppliers
inquiry
![4,7-Dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde](/CAS/20180601/GIF/1019853-65-6.gif)
1019853-65-6
7 suppliers
inquiry
Yield:1019853-65-6 76%
Reaction Conditions:
Stage #1: 4,7-dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carbonitrilewith ammonium sulphate;1,1,1,3,3,3-hexamethyl-disilazane in toluene;Reflux;
Stage #2: with diisobutylaluminium hydride in dichloromethane;toluene at -78; for 3 h;Inert atmosphere;
Stage #3: with water monomer in dichloromethane;ethyl acetate;toluene; for 2 h;
Steps:
6 Preparation 6: 4,7-Dihydro-4-oxo-2-[(triphenylmethyl)ami no]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde
Preparation 6: 4,7-Dihydro-4-oxo-2-[(triphenylmethyl)ami no]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde HMDS (6mmol, 1.3mL) was added to a mixture of 4,7-dihydro-4-oxo-2- [(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carbonitrile (1.30 g, 3 mmol) with ammonium sulphate (397 mg, 0.3mmol) in dry toluene (8mL) in around bottomed flask. A reflux condenser was fitted, and the flask was heated atreflux temperature overnight. The mixture was cooled to room temperature andconcentrated under reduced pressure. Under a positive pressure of argon, the crudereaction mixture was solubilised in dry dichloromethane (8mL) and cooled to -78°C. At this temperature, DiBAL-H (4.5 mL, 1 M in dichloromethane, 4.5 mmol) wasadded dropwise. After 2 hours, analysis by TLC (EtOAc lOO%) indicated that somestarting material remained. So, a further 2mL DiBAL-H solution was addeddropwise. After 1 hour, the reaction was complete and a mixture of H20/AcOH (9/1,3.5 mL) was added at -78 °C. The reaction mixture was allowed to warm to room temperature slowly. A mixture of EtOAc/H20 (1/1, 300 mL) was added to thereaction mixture and stirring continued at room temperature for 2 hours. The layerswere separated and the organic layer was washed with brine and the aqueouslayers were extracted with EtOAc. The combined organic fractions were dried overMg504, filtered and concentrated at reduced pressure. The crude reaction productwas filtered through a pad of silica gel eluting with EtOAc to afford a yellow solid(1.01 g, 2.38 mmol, 76 %). Procedure based on Brooks 2010 and Brooks 2012.OH (400 MHz, DMSO-d6) ? 11.82 (s, 1H), 10.63 (s, 1H), 9.99 (s, 1H), 7.54 (s, 1H),7.3 1-7.27 (m, 13H), 7.23-7.19 (m, 3H).HRMS (m/z E51): C26H18N402 EM-H]- Found 419.1508 Requires: 419.1508.
References:
WO2016/50806,2016,A1 Location in patent:Page/Page column 23; 24
![3H-Pyrrolo[2,3-d]pyrimidine-5-carbonitrile, 2-amino-4,7-dihydro-4-oxo-](/CAS/GIF/69205-79-4.gif)
69205-79-4
64 suppliers
inquiry
![4,7-Dihydro-4-oxo-2-[(triphenylmethyl)amino]-3H-pyrrolo[2,3-d]pyrimidine-5-carboxaldehyde](/CAS/20180601/GIF/1019853-65-6.gif)
1019853-65-6
7 suppliers
inquiry