
2-Thiopheneethanamine, N-(1,2,3,4-tetrahydro-5-methoxy-2-naphthalenyl)- synthesis
- Product Name:2-Thiopheneethanamine, N-(1,2,3,4-tetrahydro-5-methoxy-2-naphthalenyl)-
- CAS Number:102120-95-6
- Molecular formula:C17H21NOS
- Molecular Weight:287.42
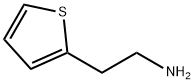
30433-91-1
341 suppliers
$5.00/5g
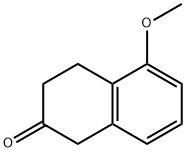
32940-15-1
308 suppliers
$13.43/250mgs:

102120-95-6
17 suppliers
inquiry
Yield:102120-95-6 98%
Reaction Conditions:
Stage #1: [2-(2-thyenyl)ethyl]amine;5-methoxy-2-tetralonewith sodium tris(acetoxy)borohydride in dichloromethane at 20;Inert atmosphere;Industry scale;
Stage #2: with acetic acid in dichloromethane at 20;
Stage #3: with sodium hydroxide in dichloromethane;water;Product distribution / selectivity;cooling with ice-water;Alkaline conditions;
Steps:
1
Preparation Example 1 Preparation of 5-methoxy-N-2'-(thien-2-yl-ethyl-tetralin-2-amine (Compound 1) To a 20 L reactor, the pre-prepared NaBH(AcO)3 (1600.62g, 7.77mol) and dichloromethane (DCM) (7.45 kg) were added at room temperature under nitrogen (N2) atmosphere, and the interior temperature was cooled to 20°C or less. In another 10 L three-necked flask, 2-thienyl ethylamine (538.8g, 4.24mol) was added to a solution of 5-methoxy-2-tetralone (622g, 3.53mol) in DCM (3.2kg) at room temperature. The mixed solution in the 10 L three-necked flask was added dropwisely to the 20 L reactor at 20°C or less. After the addition, glacial acetic acid (450g, 7.5mol) was added dropwisely thereto and then the mixture was stirred at room temperature overnight. The reaction was quenched with water under ice water bath. The pH of the reaction mixture was adjusted to be alkaline by dropwise addition of 50% aqueous sodium hydroxide (NaOH) solution. The organic phase was separated out. The water layer was extracted with DCM, and the organic phases were combined and concentrated to give 5-methoxy-N-2'-(thien-2-yl-)ethyl-tetralin-2-amine with a chemical purity of 96% and a yield of 98%.
References:
EP2476676,2012,A1 Location in patent:Page/Page column 6