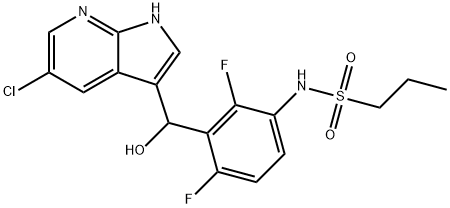
N-(3-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)(hydroxy)Methyl)-2,4-difluorophenyl)propane-1-sulfonaMide synthesis
- Product Name:N-(3-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)(hydroxy)Methyl)-2,4-difluorophenyl)propane-1-sulfonaMide
- CAS Number:1021854-28-3
- Molecular formula:C17H16ClF2N3O3S
- Molecular Weight:415.84
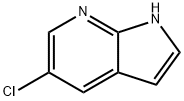
866546-07-8
237 suppliers
$6.00/1g
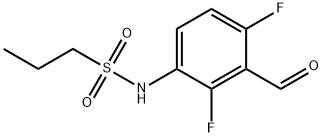
918523-58-7
36 suppliers
$205.00/250mg
![N-(3-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)(hydroxy)Methyl)-2,4-difluorophenyl)propane-1-sulfonaMide](/CAS/GIF/1021854-28-3.gif)
1021854-28-3
2 suppliers
inquiry
Yield:1021854-28-3 88%
Reaction Conditions:
with potassium carbonate in methanol;water at 130; for 0.5 h;Microwave irradiation;
Steps:
N-(3-((5-Chloro-1H-pyrrolo[2,3-b]pyridine-3-yl)(hydroxyl)methyl)2,4-difluorophenyl)propane-1-sulfonamide (7a).
Potassium carbonate (157 mg, 1.14 mmol) was added to a solution of 5 (47 mg, 0.175 mmol) and 5-chloro-7-azaindole (6a) (32 mg, 0.210 mmol) in methanol:water (1:1; 6.0 mL). The reaction mixture was microwaved at 130 °C for 30 min. The reaction mixture pH was then adjusted to 7 with 4.0 N HCl and extracted with ethyl acetate (3 x 10 mL). The combined organics were then dried (MgSO4), filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel afford 7a (64 mg, 88%) as a light-brown solid.
References:
Buck, Jason R.;Saleh, Sam;Imam Uddin, Md.;Manning, H. Charles [Tetrahedron Letters,2012,vol. 53,# 32,p. 4161 - 4165] Location in patent:supporting information; experimental part
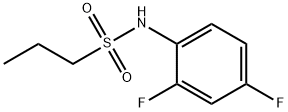
918523-57-6
20 suppliers
$173.40/250mg
![N-(3-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)(hydroxy)Methyl)-2,4-difluorophenyl)propane-1-sulfonaMide](/CAS/GIF/1021854-28-3.gif)
1021854-28-3
2 suppliers
inquiry
367-25-9
487 suppliers
$5.00/5g
![N-(3-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)(hydroxy)Methyl)-2,4-difluorophenyl)propane-1-sulfonaMide](/CAS/GIF/1021854-28-3.gif)
1021854-28-3
2 suppliers
inquiry