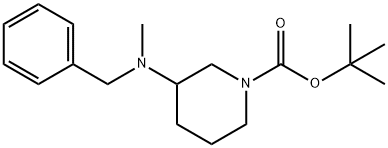
TERT-BUTYL 3-(N-BENZYL-N-METHYLAMINO) PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-(N-BENZYL-N-METHYLAMINO) PIPERIDINE-1-CARBOXYLATE
- CAS Number:1027345-51-2
- Molecular formula:C18H28N2O2
- Molecular Weight:304.43
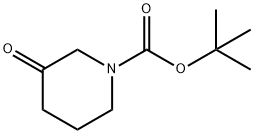
98977-36-7
420 suppliers
$6.00/1g
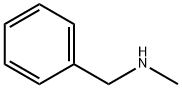
103-67-3
422 suppliers
$5.00/10g

1027345-51-2
11 suppliers
inquiry
Yield:1027345-51-2 69%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 20;Inert atmosphere;
Steps:
General Procedure A for the Preparation of Intermediates 3a-d by Reductive Amination
General procedure: The corresponding cyclic ketone (2a-d) (1 eq.) was dissolved in dry DCM. Glacial AcOH(1.1-1.15 eq.) and N,N-benzylmethylamine (1.1-1.25 eq.) were added, followed by portion-wiseaddition of Na(OAc)3BH (1.5-1.6 eq.). The mixture was stirred at rt, under N2 atmosphere and overNa2SO4 (0.5-1 g) overnight. In case of incomplete consumption of the ketone, additional amine andreductive agent were added to the mixture. After complete conversion, saturated NaHCO3 solutionwas added and phases were separated. The aqueous layer was extracted thrice with DCM. Combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. The residue waspurified by flash column chromatography
References:
Andreev, Stanislav;Pantsar, Tatu;Ansideri, Francesco;Kudolo, Mark;Forster, Michael;Schollmeyer, Dieter;Laufer, Stefan A.;Koch, Pierre [Molecules,2019,vol. 24,# 12,art. no. 2331]