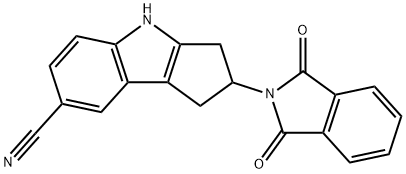
Cyclopent[b]indole-7-carbonitrile, 2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)-1,2,3,4-tetrahydro- synthesis
- Product Name:Cyclopent[b]indole-7-carbonitrile, 2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)-1,2,3,4-tetrahydro-
- CAS Number:1029691-07-3
- Molecular formula:C20H13N3O2
- Molecular Weight:327.34

1029691-06-2
32 suppliers
$60.00/100mg
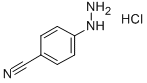
2863-98-1
269 suppliers
$6.00/1g
![Cyclopent[b]indole-7-carbonitrile, 2-(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)-1,2,3,4-tetrahydro-](/CAS/GIF/1029691-07-3.gif)
1029691-07-3
3 suppliers
inquiry
Yield:1029691-07-3 66%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;acetic acid at 90 - 100; for 36 h;
Steps:
2
Mix (+/-)-2-(3-oxo-cyclopentyl)-isoindole-1,3-dione (12.7 g, 55.3 mmol) and 4-cyanophenylhydrazine-HCl (8.53 g, 50.3 mmol) in HOAc (200 mL) and 4N HCl dioxane (50 mL). Using mechanical stirring, heat the reaction to 90° C. for 18 h, then add additional 4N HCl dioxane (20 mL). Heat the reaction to 100° C. for 18 h. Dilute the reaction mixture with water (600 mL) and collect a black solid by vacuum filtration. Sonicate the solid with MeOH (200 mL), then collect and dry in a vacuum oven to give 10.94 g (66%) of a gray-brown solid. MS (m/z): 328 (M+1), 326 (M-1).
References:
US2010/69404,2010,A1 Location in patent:Page/Page column 12