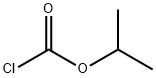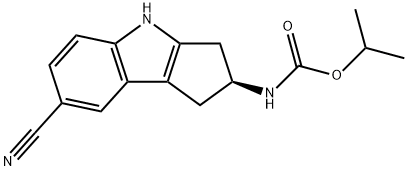
(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester synthesis
- Product Name:(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester
- CAS Number:1029691-23-3
- Molecular formula:C16H17N3O2
- Molecular Weight:283.33
Yield:1029691-23-3 86%
Reaction Conditions:
Stage #1: ((S)-7-Cyano-1,2,3,4-tetrahydro-cyclopenta[b]indol-2-yl)-carbamic acid tert-butyl esterwith hydrogenchloride in 1,4-dioxane at 20; for 18 h;
Stage #2: isopropyl chloroformatewith N-ethyl-N,N-diisopropylamine in dichloromethane;toluene at 20; for 4 h;
Steps:
7
PREPARATION 7((S)-7-Cyano-1,2,3,4-tetrahydro-cyclopenta[b]indol-2-yl)-carbamic acid isopropyl ester ((S)-7-Cyano-1,2,3,4-tetrahydro-cyclopenta[b]indol-2-yl)-carbamic acid tert-butyl ester (10 g, 33.63 mmol) is dissolved in 1,4-dioxane (102 mL) and treated with 4 M HCl/dioxane (102 mL) at room temperature. After 18 h a solid is filtered off and washed with Et2O (50 mL) and then dried in vacuo.The solid is slurried in dichloromethane (168 mL) and treated with diisopropylethylamine (12.3 mL, 70.1 mmol) and isopropyl chloroformate (1.0 M in toluene, 34.0 mL, 34.0 mmol) at room temperature. After 4 h the reaction is treated with water (50 mL) and concentrated to give an aqueous slurry of the product. The reaction is further diluted with water (500 mL) and sonicated for 15 min in an ultrasonic bath. A tan solid is filtered off and dried in vacuo at 40° C. The solid is slurried in Et2O (100 mL), sonicated for 10 min in an ultrasonic bath, filtered, washed with Et2O (50 mL), and then dried in vacuo to give 8.20 g (86%) of the title compound as a tan solid. LC-ES/MS m/z 284 [M+H]+, 282 [M-H]-, TR=2.20 min; 1H-NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 7.78 (s, 1H), 7.52 (d, J=7.5 Hz, 1H), 7.39 (d, J=8.4 Hz, 1H), 7.28 (dd, J=1.8, 8.4 Hz, 1H), 4.77-4.63 (m, 2H), 3.18-3.04 (m, 2H), 2.70 (dd, J=6.2, 15.8 Hz, 1H), 2.58 (dd, J=6.2, 14.5 Hz, 1H), 1.13 (d, J=6.2 Hz, 6H).
References:
US2011/118326,2011,A1 Location in patent:Page/Page column 9-10
930-30-3
263 suppliers
$10.00/1g
![(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester](/CAS/GIF/1029691-23-3.gif)
1029691-23-3
5 suppliers
inquiry

1029691-06-2
31 suppliers
$60.00/100mg
![(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester](/CAS/GIF/1029691-23-3.gif)
1029691-23-3
5 suppliers
inquiry
![2-(7-BROMO-1,2,3,4-TETRAHYDROCYCLOPENTA[B]INDOL-2-YL)ISOINDOLINE-1,3-DIONE](/CAS/20180703/GIF/1196037-57-6.gif)
1196037-57-6
0 suppliers
inquiry
![(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester](/CAS/GIF/1029691-23-3.gif)
1029691-23-3
5 suppliers
inquiry
![7-bromo-1,2,3,4-tetrahydrocyclopenta[b]indol-2-amine](/CAS/20150408/GIF/1196037-58-7.gif)
1196037-58-7
13 suppliers
inquiry
![(S)-(7-Cyano-1,2,3,4-tetrahydrocyclopenta[b]indol-2-yl)carbamic Acid Isopropyl Ester](/CAS/GIF/1029691-23-3.gif)
1029691-23-3
5 suppliers
inquiry
![Carbamic acid, N-[(2S)-7-cyano-1,2,3,4-tetrahydrocyclopent[b]indol-2-yl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1029691-25-5.gif)