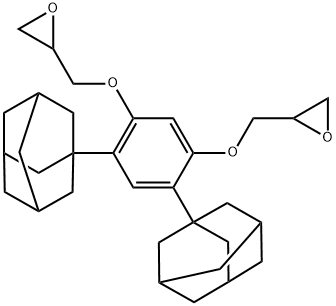
Oxirane, 2,2'-[[4,6-bis(tricyclo[3.3.1.13,7]dec-1-yl)-1,3-phenylene]bis(oxyMethy synthesis
- Product Name:Oxirane, 2,2'-[[4,6-bis(tricyclo[3.3.1.13,7]dec-1-yl)-1,3-phenylene]bis(oxyMethy
- CAS Number:1030386-18-5
- Molecular formula:C32H42O4
- Molecular Weight:490.67
Yield:1030386-18-5 92%
Reaction Conditions:
with sodium hydroxide in dimethyl sulfoxide;4-methyl-2-pentanone at 45;
Steps:
2
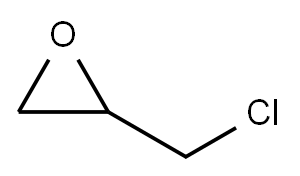
Example 2; Synthesis of 4,6-bis(1-adamantyl)-1,3-digylcidyloxybenzene A 500 mL four-necked flask equipped with a reflux condenser, a stirrer, a thermometer, and a nitrogen inlet tube was charged with 57 mL of MIBK, 157 mL of DMSO, and 98 g (1.057 mol) of epichlorohydrin and replaced with nitrogen for 30 minutes. To this solution, 52.01 g (0.137 mol) of 4,6-bis(1-adamantyl)-1,3-dihydroxybenzene synthesized in Example 1 was added, and the flask was replaced with nitrogen for 30 minutes and then heated at 45°C while stirring. This solution was added with 11.6 g (0.290 mol) of sodium hydroxide over 0.5 hour and the solution was stirred for 1.5 hours. Then, 2.9 g (0.0725 mol) of sodium hydroxide was added and the solution was further stirred for 1 hour. The reaction mixture was cooled to room temperature, and 300 mL of chloroform was added. After washing with 500 mL of water, an aqueous 0.1 mol/L HCl solution was added to the mixture and the organic layer was separated. After further washing with water until the aqueous phase became neutral, the organic layer was concentrated and dried until the weight became constant in a reduced pressure drier at 100°C to obtain 4,6-bis(1-adamantyl)-1,3-diglycidyloxybenzene as a pale-yellow solid (yield: 92%, LC purity: 99.20%, epoxy equivalent: 267, melting point: 193°C). The obtained 4,6-bis(1-adamantyl)-1,3-diglycidyloxybenzene, was identified by nuclear magnetic resonance spectrum (1H-NMR and 13C-NMR). The spectrum data are shown below. The nuclear magnetic resonance spectrum was measured using chloroform-d as a solvent and JNM-ECA500 manufactured by JEOL Ltd. as a measurement apparatus. 1H-NMR (500 MHz): 1.77 (s, 12H), 2.08 (s, 20H), 2.80 (dd, 2H), 2.91 (dd, 1H), 3.39 (m, 2H), 3.98 (dd, 2H), 4.24 (dd, 2H), 6.47 (s, 1H), 7.08 (s, 1H) 13C-NMR (125 MHz): 29.2, 36.7, 37.2, 41.1, 44.7, 50.5, 69.0, 99.6, 125.0, 130.5, 155.9
References:
EP2090563,2009,A1 Location in patent:Page/Page column 18
![1,3-Benzenediol, 4,6-bis(tricyclo[3.3.1.13,7]dec-1-yl)-](/CAS/20210305/GIF/367279-75-2.gif)