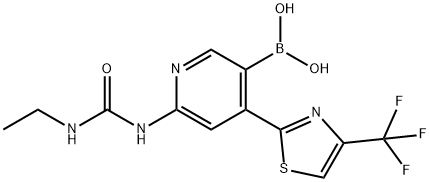
6-(3-ethylureido)-4-(4-(trifluoroMethyl)thiazol-2-yl)pyridin-3-ylboronic acid synthesis
- Product Name:6-(3-ethylureido)-4-(4-(trifluoroMethyl)thiazol-2-yl)pyridin-3-ylboronic acid
- CAS Number:1031432-91-3
- Molecular formula:C12H12BF3N4O3S
- Molecular Weight:360.12
Yield:-
Reaction Conditions:
Stage #1: N-{5-bromo-4-[4-(trifluoromethyl)-1,3-thiazol-2-yl]pyridin-2-yl}-N′-ethylurea;bis(pinacol)diboranewith potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in dimethyl sulfoxide at 90; for 5 h;Inert atmosphere;Microwave irradiation;
Stage #2: with hydrogenchloride;water in dimethyl sulfoxide at 100;
Stage #3: with sodium hydroxide at 18 - 26; pH=5.5;
Steps:
12
Intermediate 121-Ethyl-3-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-4-(4-(trifluoromethyl)thiazol-2-yl)pyridin-2-yl)urea1-(5-Bromo-4-(4-(trifluoromethyl)thiazol-2-yl)pyridin-2-yl)-3-ethylurea (Intermediate 13, 200 mg, 0.51 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(1,3,2-dioxaborolane) (386 mg, 1.52 mmol), potassium acetate (149 mg, 1.52 mmol), and 1,1'-bis(diphenylphosphino)ferrocene-palladium dichloride (20.72 mg, 0.03 mmol) were taken in a microwave vial and degassed with argon. DMSO (4 mL) was added to the vial and the solution was heated at 90° C. for 5 h. The reaction mixture was partitioned between water and ethyl acetate. The layers were separated and the organic layer was back extracted three times with ethyl acetate. The organic layers were combined and washed with water and brine, then dried over magnesium sulfate and concentrated under reduced pressure to give a light brown solid that was a mixture of 1-Ethyl-3-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-4-(4-(trifluoromethyl)thiazol-2-yl)pyridin-2-yl)urea (Intermediate 12A, 35%), {6-{[(ethylamino)carbonyl]amino}-4-[4-(trifluoromethyl)-1,3-thiazol-2-yl]pyridin-3-yl}boronic acid (25%) and N-ethyl-N'-{4-[4-(trifluoromethyl)-1,3-thiazol-2-yl]pyridin-2-yl}urea (25%). The crude mixture was taken to the next step without further purification.LC/MS (ES+)[(M+H)+]: 361 for C12HuBF3N4O3S. 1H NMR (300 MHz, d6-DMSO): 1.09 (t, 3H), 3.12 (m, 2H), 7.82 (t, 1H), 7.95 (s, 1H), 8.17 (s, 2H), 8.31 (s, 1H), 8.65 (s, 1H), 9.35 (s, 1H).In a subsequent step, the crude solid mixture was suspended in 2 L of water and 500 mL of 6N HCl, and heated to 100° C. Upon hydrolysis, the boronic acid dissolves in water at that temperature. Insoluble orange-yellow colored material was observed. This was removed by filtering the hot reaction mixture (Buchner funnel). The hydrolysis was monitored by LC/MS, cooled to room temperature, and adjusted to pH=5.5 with 20% NaOH. The pure boronic acid precipitated from the solution. The product (Intermediate 12) was filtered, washed with water, and then dried in vacuo at 100° C.MS (ESP): 361 (MH+) for C12H12BF3N4O3S1H NMR (d6-DMSO): δ 1.11 (t, 3H), 3.16-3.22 (q, 2H), 7.71 (bt, 1H), 7.89 (d, 1H), 8.16 (s, 1H), 8.28 (s, 1H), 8.63 (s, 1H), 9.26 (s, 1H).
References:
US2010/317624,2010,A1 Location in patent:Page/Page column 47-48
![5-bromo-2-[(ethylcarbamoyl)amino]pyridine-4-carbothioamide](/CAS/20200331/GIF/1031432-71-9.gif)
1031432-71-9
0 suppliers
inquiry

1031432-91-3
0 suppliers
inquiry
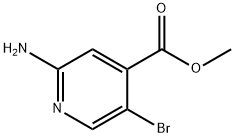
882499-87-8
146 suppliers
$47.00/1g

1031432-91-3
0 suppliers
inquiry
![1-{5-bromo-4-[4-hydroxy-4-(trifluoromethyl)-4,5-dihydro-1,3-thiazol-2-yl]pyridin-2-yl}-3-ethylurea](/CAS/20200331/GIF/1031432-67-3.gif)
1031432-67-3
0 suppliers
inquiry

1031432-91-3
0 suppliers
inquiry
![5-bromo-2-[(ethylcarbamoyl)amino]pyridine-4-carboxamide](/CAS/20200119/GIF/1031432-73-1.gif)
1031432-73-1
0 suppliers
inquiry

1031432-91-3
0 suppliers
inquiry
![1-{5-bromo-4-[4-(trifluoromethyl)-1,3-thiazol-2-yl]pyridin-2-yl}-3-ethylurea](/CAS/20200331/GIF/1031432-65-1.gif)
