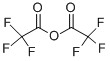
4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester synthesis
- Product Name:4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester
- CAS Number:1034975-53-5
- Molecular formula:C23H32F3N3O4
- Molecular Weight:471.51
Yield:1034975-53-5 78%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 4 h;Inert atmosphere;
Steps:
1.e e) Synthesis of tert-butyl 4-(4-methylpiperazin- 1-yl)-2-(2,2,2-trifluoro-N-(tetrahydropyran-4-yl)-acetamido) benzoate
Under nitrogen atmosphere tert-butyl 4-(4-methylpiperazin- 1 -yl)-2- (tetrahydropyran-4-ylamino)benzoate (0.69 mmol, 260 mg) was dissolved in DCM (7 ml) and cooled to 0°C. Then TEA (1.1 mmol, 154 tl) was added followed by a slow additionof TFAA (0.9 mmol, 253 tl). Reaction was terminated after 4 hours, washed with water, diluted with DCM, washed with a saturated solution of aqueous NaHCO3, brine solution, dried over sodium sulfate, filtered and evaporated to dryness affording the title compound as an orange foam (253 mg, 78%).1H NMR (400 MHz, CDC13) ? 7.95 (d, J 8.9 Hz, 1H), 6.91 (dd, J 8.9, 2.6 Hz, 1H), 6.55 (d, J= 2.4 Hz, 1H), 4.64 (ddd, J= 12.1, 8.1, 4.0 Hz, 1H), 4.05 - 3.95 (m, 2H),3.90 (dd, J 11.6, 4.3 Hz, 1H), 3.64-3.32 (m, 6H), 2.81 (s, 3H), 2.52 (dd, J= 16.0, 9.9 Hz, 4H), 2.12 (d, J= 12.7 Hz, 1H), 1.79 - 1.32 (m, 1OH), 1.32 - 1.02 (m, 2H).
References:
WO2016/96709,2016,A1 Location in patent:Page/Page column 46; 47
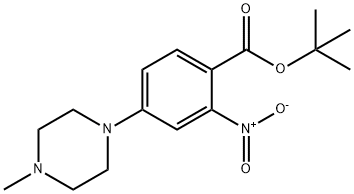
942271-61-6
55 suppliers
inquiry
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester](/CAS/20180703/GIF/1034975-53-5.gif)
1034975-53-5
13 suppliers
inquiry

1034975-35-3
96 suppliers
inquiry
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester](/CAS/20180703/GIF/1034975-53-5.gif)
1034975-53-5
13 suppliers
inquiry
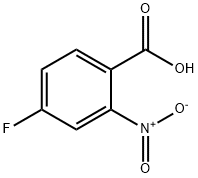
394-01-4
252 suppliers
$8.00/5g
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester](/CAS/20180703/GIF/1034975-53-5.gif)
1034975-53-5
13 suppliers
inquiry
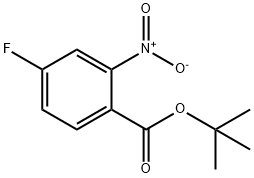
942271-60-5
60 suppliers
inquiry
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)(2,2,2-trifluoroacetyl)amino]benzoic acid tert-butyl ester](/CAS/20180703/GIF/1034975-53-5.gif)
1034975-53-5
13 suppliers
inquiry
![4-(4-methylpiperazin-1-yl)-2-[(tetrahydropyran-4-yl)amino]benzoic acid tert-butyl ester](/CAS/20180629/GIF/1034975-40-0.gif)