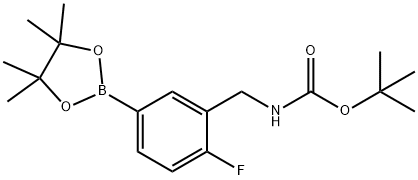
3-(Boc-aMinoMethyl)-4-fluorobenzeneboronic acid pinacol ester, 96% synthesis
- Product Name:3-(Boc-aMinoMethyl)-4-fluorobenzeneboronic acid pinacol ester, 96%
- CAS Number:1035391-48-0
- Molecular formula:C18H27BFNO4
- Molecular Weight:351.22
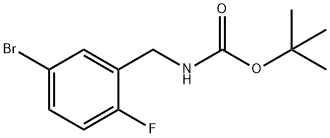
491836-84-1
7 suppliers
inquiry

73183-34-3
564 suppliers
$6.00/5g

1035391-48-0
11 suppliers
$159.00/250mg
Yield:1035391-48-0 84%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 22; for 1.66667 h;Heating / reflux;
Steps:
113.113A
EXAMPLE 113; 4-(4-(3-(aminomethyl)-4-fluorophenyl)-lH-benzordlimidazol-2-yl)-l-(7H-pyrrolor2,3- dlpyrimidin-4-yl)piperidin-4-amine113A. tert-butyl 2-fluoro-5-(4A5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)benzylcarbamateBis(pinacolato)diboron (2.83 g, 11.13 mmol), tert-butyl 5-bromo-2-fluorobenzylcarbamate (2.82 g, 9.27 mmol) (CAS no. 491836-84-1, prepared by a method very similar to that described in WO 2005094822) and potassium acetate (4.55 g, 46.36 mmol) were suspended in 1,4-dioxane (25 mL) at 22 0C under nitrogen. A stream of nitrogen was passed through the mixture for 10 minutes. l,r-Bis(diphenylphosphino)ferrocene- palladium dichloride (0.191 g, 0.23 mmol) was added and the mixture was heated at reflux for 1.5 hours. The mixture was concentrated and the residue was treated with DCM (500 mL). The insoluble material was removed by filtration through a plug of silica. The silica
References:
WO2008/75109,2008,A1 Location in patent:Page/Page column 232-233