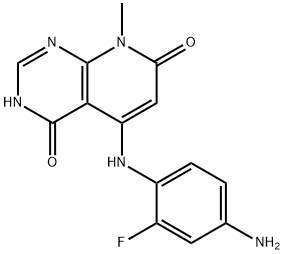
5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE synthesis
- Product Name:5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE
- CAS Number:1035556-26-3
- Molecular formula:C14H12FN5O2
- Molecular Weight:301.28
![3-BENZYL-5-(2-FLUORO-4-NITROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-25-2.gif)
1035556-25-2
4 suppliers
inquiry
![5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-26-3.gif)
1035556-26-3
4 suppliers
inquiry
Yield:1035556-26-3 65%
Reaction Conditions:
with ammonium formate;palladium hydroxide on carbon in 1,4-dioxane at 90; for 4 h;
Steps:
5
5-(4-Amino-2-fluorophenylamino)-8-methylpyrido [2,3-d] pyrimidine- 4,7(3H,8H)-dione (compound 5E): 3-Benzyl-5-(2-fluoro-4-nitrophenylamino)-8- methylpyrido[2,3- d]pyrimidine-4,7(3H,8H)-dione (900 mg, 2.1 mmol), palladium hydroxide (20% on carbon, 500 mg) and ammonium formate (500 mg, 7.9 3 mmol, 3.7 eq) were stirred in dioxane (10 mL) at 90 0C for 4 hours. The suspension was filtered and the solid refluxed in DMF (20 mL) for 1 hour then filtered. This was repeated 3 times. The filtrates were combined and evaporated to give 420 mg of title compound as a white solid (65%). 1H NMR (400 MHz, MeOD) δ ppm 3.56 (br. s., 3 H) 5.38 (s, 1 H) 6.51 (s, 1 H) 6.54 (t, J=I.89 Hz, 1 H) 7.07 (t, J=8.46 Hz, 1 H) 8.17 (s, 1 H) [M+H] calc'd for Ci4Hi2FN5O2, 302; found, 302.
References:
WO2008/79814,2008,A2 Location in patent:Page/Page column 107-108
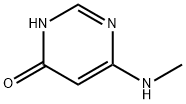
1122-67-4
26 suppliers
inquiry
![5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-26-3.gif)
1035556-26-3
4 suppliers
inquiry
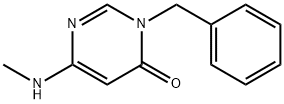
1035556-22-9
6 suppliers
inquiry
![5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-26-3.gif)
1035556-26-3
4 suppliers
inquiry
![3-Benzyl-5-hydroxy-8-methyl-3H,8H-pyrido[2,3-d]pyrimidine-4,7-dione](/CAS/20180601/GIF/1035556-23-0.gif)
1035556-23-0
6 suppliers
inquiry
![5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-26-3.gif)
1035556-26-3
4 suppliers
inquiry
![3-Benzyl-5-chloro-8-methyl-3H,8H-pyrido[2,3-d]pyrimidine-4,7-dione](/CAS/20180529/GIF/1035556-24-1.gif)
1035556-24-1
5 suppliers
inquiry
![5-(4-AMINO-2-FLUOROPHENYLAMINO)-8-METHYLPYRIDO[2,3-D]PYRIMIDINE-4,7(3H,8H)-DIONE](/CAS/20180529/GIF/1035556-26-3.gif)
1035556-26-3
4 suppliers
inquiry