
4(1H)-Quinazolinone, 8-methoxy-2-(4-methoxyphenyl)- (9CI) synthesis
- Product Name:4(1H)-Quinazolinone, 8-methoxy-2-(4-methoxyphenyl)- (9CI)
- CAS Number:1036-50-6
- Molecular formula:C16H14N2O3
- Molecular Weight:0
![Benzamide, 3-methoxy-2-[(4-methoxybenzoyl)amino]-](/CAS/20210305/GIF/211172-80-4.gif)
211172-80-4
0 suppliers
inquiry

1036-50-6
2 suppliers
inquiry
Yield:1036-50-6 77%
Reaction Conditions:
with sodium hydroxide in water at 60; for 6 h;
Steps:
8-Methoxy-2-(4-methoxyphenyl)quinazolin-4-one (57c).
8-Methoxy-2-(4-methoxyphenyl)quinazolin-4-one (57c). 3-Methoxy-2-(4-methoxy- benzamido)benzamide 56c (378 mg, 1.3 mmol) was heated with aqueous sodium hydroxide (0.5 M, 80 mL) at 60°C for 6 h. The mixture was acidified by addition of aqueous hydrochloric acid (9 M) to pH 2. The precipitate was collected by filtration, washed (water) and dried to give 8-methoxy-2-(4-methoxyphenyl)quinazolin-4-one 57c (272 mg, 77%) as a white solid: mp 230-232°C (lit.81 226-228°C); 1H NMR ((CD3)2SO) δ 3.84 (3 H, s, Ph-OMe), 3.94 (8-OMe), 7.09 (2 H, m, Ph 3,5-H2), 7.35 (1 H, dd, J = 8.0, 1.0 Hz, 7-H), 7.40 (1 H, t, J = 8.0 Hz, 6-H), 7.68 (1 H, dd, J = 8.0, 1.0 Hz, 5-H), 8.18 (2 H, m, Ph 2,6-H2), 12.41 (1 H, s, NH); 13C NMR ((CD3)2SO) (HSQC / HMBC) δ 55.45 (Ph-OMe), 1 13.96 (Ph 3,5-C2), 1 15.1 1 (7-C), 1 16.88 (5-C), 121.69 (4a-C), 125.09 (Ph 1 - C), 126.34 (6-C), 129.40 (Ph 2,6-C2), 139.10 (8a-C), 150.50 (2-C), 154.54 (8-C), 161.74 (Ph 4-C), 162.25 (4-C).
References:
WO2014/87165,2014,A1 Location in patent:Page/Page column 120

106782-78-9
38 suppliers
$80.00/5mg

1036-50-6
2 suppliers
inquiry
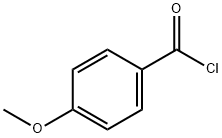
100-07-2
388 suppliers
$35.00/25g

1036-50-6
2 suppliers
inquiry
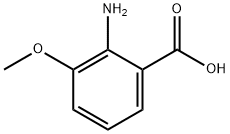
3177-80-8
258 suppliers
$9.00/1g

1036-50-6
2 suppliers
inquiry