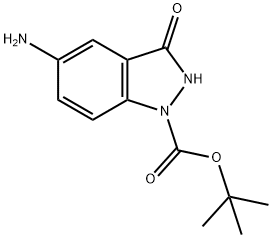
TERT-BUTYL 5-AMINO-3-OXO-2,3-DIHYDRO-1H-INDAZOLE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 5-AMINO-3-OXO-2,3-DIHYDRO-1H-INDAZOLE-1-CARBOXYLATE
- CAS Number:1036389-72-6
- Molecular formula:C12H15N3O3
- Molecular Weight:249.27

876343-67-8
10 suppliers
inquiry

1036389-72-6
8 suppliers
inquiry
Yield:1036389-72-6 90%
Reaction Conditions:
with hydrogen;palladium on activated carbon in tetrahydrofuran at 20; under 760.051 Torr; for 2 h;
Steps:
19.D
19C (0.100 g, 0.358 mmol) was partially dissolved in THF (5 mL) and water (0.6 mL), then the mixture was degassed (3 x Ar/vacuum). To this solution, Pd- C (10 % wt) (0.057 g, 0.054 mmol) was added. The mixture was degassed again (3 x Ar) and allowed to stir at rt under hydrogen (1 atm) for 2 h. The Pd-C was filtered and the solvent was removed under reduced pressure to give 19D (0.080 g, 0.321 mmol, 90 % yield) as a yellowish glass. LC-MS: (Phenom. Luna C18 30x4.6mm 5u; sol. A 10% MeCN - 90% H2O - 0.1% TFA; sol. B 90% MeCN - 10% H2O - 0.1% TFA; wavelength 220 nm; flow rate 5 mL/min; gradient time 2 min; start % B = 0%, final % B = 100%) 0.562 min, [M+l]+ = 250.1. Purity >95% 1H-NMR: (400 MHz, DMSO-d6) δ ppm 1.55 (s, 9 H) 5.15 (s, 2 H) 6.73 (d, J=2.20 Hz, 1 H) 6.86 (dd, J=8.79, 2.20 Hz, 1 H) 7.63 (d, J=7.70 Hz, 1 H).
References:
WO2008/79759,2008,A1 Location in patent:Page/Page column 131