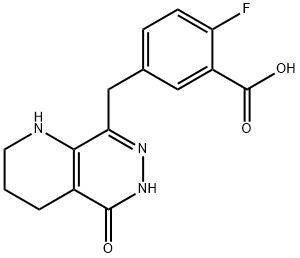
Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]- synthesis
- Product Name:Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-
- CAS Number:1036403-32-3
- Molecular formula:C15H14FN3O3
- Molecular Weight:303.29
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-, methyl ester](/CAS/20210111/GIF/1036403-28-7.gif)
1036403-28-7
0 suppliers
inquiry
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-](/CAS/20210111/GIF/1036403-32-3.gif)
1036403-32-3
1 suppliers
inquiry
Yield:1036403-32-3 85%
Reaction Conditions:
Stage #1: methyl 2-fluoro-5-[(5-oxo-1,2,3,4,5,6-hexahydropyrido[2,3-d]pyridazin-8-yl)methyl]benzoatewith lithium hydroxide in tetrahydrofuran;water at 50;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20; pH=4;Inert atmosphere;
Steps:
6.3.2. 2-Fluoro-5-[(5-oxo-1,2,3,4,5,6-hexahydropyrido[2,3-d]pyridazin-8-yl)methyl]benzoic acid (19)
Compound 18 (2.8 g, 8.8 mmol) was dissolved in hot THF (150 mL), and was treated with LiOH (253 mg, 10.6 mmol) in water (20 mL) at 50 °C overnight. After cooling to room temperature, the reaction mixture was acidified with diluted HCl to a pH4, and concentrated to about 10 mL. The formed solid material was collected by filtration, washed with water and dried to provide 19. Yield: 2.4 g (85%). MS (ESI) m/z 304 (M+H)+; 1H NMR (300 MHz, DMSO-d6): δ 1.61-1.77 (m, 2H), 2.34 (t, J = 6.10 Hz, 2H), 3.06-3.25 (m, 2H), 3.84 (s, 2H), 6.36 (s, 1H), 7.22 (dd, J = 10.85, 8.48 Hz, 1H), 7.39-7.52 (m, 1H), 7.73 (dd, J = 7.12, 2.37 Hz, 1H), 11.82 (s, 1H) 13.19 (s, 1H).
References:
Zhu, Gui-Dong;Gong, Jianchun;Gandhi, Viraj B.;Liu, Xuesong;Shi, Yan;Johnson, Eric F.;Donawho, Cherrie K.;Ellis, Paul A;Bouska, Jennifer J.;Osterling, Donald J.;Olson, Amanda M.;Park, Chang;Luo, Yan;Shoemaker, Alexander;Giranda, Vincent L.;Penning, Thomas D. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 15,p. 4635 - 4645] Location in patent:experimental part

1036402-42-2
1 suppliers
inquiry
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-](/CAS/20210111/GIF/1036403-32-3.gif)
1036403-32-3
1 suppliers
inquiry
![Pyrido[2,3-d]pyridazin-5(6H)-one, 8-[(3-bromo-4-fluorophenyl)methyl]-](/CAS/20210111/GIF/1036402-39-7.gif)
1036402-39-7
0 suppliers
inquiry
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-](/CAS/20210111/GIF/1036403-32-3.gif)
1036403-32-3
1 suppliers
inquiry
![Benzoic acid, 5-[(5,6-dihydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-2-fluoro-, methyl ester](/CAS/20210111/GIF/1036403-16-3.gif)
1036403-16-3
0 suppliers
inquiry
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-](/CAS/20210111/GIF/1036403-32-3.gif)
1036403-32-3
1 suppliers
inquiry
605-38-9
193 suppliers
$14.00/5g
![Benzoic acid, 2-fluoro-5-[(1,2,3,4,5,6-hexahydro-5-oxopyrido[2,3-d]pyridazin-8-yl)methyl]-](/CAS/20210111/GIF/1036403-32-3.gif)
1036403-32-3
1 suppliers
inquiry