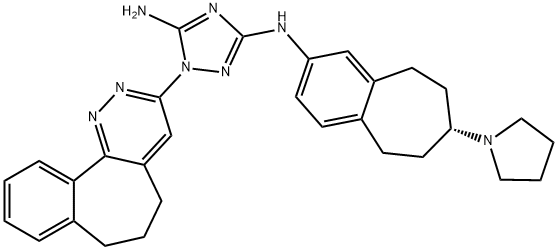
(R)-1-(6,7-Dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl)-1H-1,2,4-triazole-3,5-diamine synthesis
- Product Name:(R)-1-(6,7-Dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl)-1H-1,2,4-triazole-3,5-diamine
- CAS Number:1037624-76-2
- Molecular formula:C30H34N8
- Molecular Weight:506.64
Yield:1037624-76-2 50%
Reaction Conditions:
in isopropyl alcohol at 90; for 18 h;
Steps:
2
SYNTHETIC EXAMPLE 2. Synthesis of 1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1 ,2-c]pyridazin-3-yl)-N3-(7-(S)-(pyrrolidin-1 -yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)-1 H- 1 ,2,4-triazole-3,5- diamine and 1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1 ,2-c]pyridazin-3-yl)-N3-(7-(R)- (pyrrolidin-1 -yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)-1 H- 1 ,2,4-triazole-3,5- diamine. Phenyl (S)-N'-cyano-N-(7-(pyrrolidin-1-yl)-6, 7,8, 9-tetrahydro-5H- benzo[7]annulene-2-yl)carbamimidate and phenyl (R)-N'-cyano-N-(7-(pyrrolidin-1-yl)- 6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)carbamimidate were treated with 3-hydrazino-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazine in a similar manner as described above in Synthetic Example 2 to yield 1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1 ,2-c]pyridazin-3-yl)-N3-(7-(S)-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro- 5H-benzo[7]annulene-2-yl)-1H-1 ,2,4-triazole-3,5-diamine and 1-(6,7-dihydro-5H- benzo[6,7]cyclohepta[1 ,2-c]pyridazin-3-yl)-N3-(7-(R)-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro- 5H-benzo[7]annulene-2-yl)-1H-1 ,2,4-triazole-3,5-diamine . For example, a suspension of a single enantiomer of phenyl N'-cyano-N-(7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H- benzo[7]annulen-2-yl)carbamimidate (0.06 g, 0.16 mmol) and 3-hydrazino-6,7-dihydro- 5H-benzo[6,7]cyclohepta[1,2-c]pyridazine (0.036 g, 0.16 mmol) in 1 ml. of IPA was stirred at 90 °C for 18 h. After cooling to ambient temperature, the solvent was evaporated and the residue was purified by HPLC to provide enantiomerically pure 1- (6,7-dihydro-5H-benzo[6,7]cyclohepta[1 ,2-c]pyhdazin-3-yl)-N3-(7-(R)-(pyrrolidin-1-yl)- 6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)-1 H-1 ,2,4-triazole-3,5-diamine as a yellow solid (0.04 g, 50%). 1H NMR (DMSO-d6, 300 MHz) 9.11 (s, 1 H), 7.94 (s, 1 H), 7.85 (br s, 2H), 7.72 (m, 1 H), 7.46 (m, 3H), 7.37 (m, 2H), 7.06 (m, 1 H), 3.46 (m, 4H), 3.12 (m, 2H), 2.72 (m, 4H), 2.61 (m, 3H), 2.25 (m, 5H), 2.09 - 1.82 (m, 5H) ppm; MS (ES) 507 (M+H).
References:
WO2008/83367,2008,A2 Location in patent:Page/Page column 141
![Phenyl N-cyano-N′-[(7R)-6,7,8,9-tetrahydro-7-(1-pyrrolidinyl)-5H-benzocyclohepten-2-yl]carbamimidate](/CAS/20200515/GIF/1037627-98-7.gif)
![3-hydrazinyl-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazine](/CAS/20180703/GIF/802598-74-9.gif)