
Methyl5-oxo-3,5-dihydro-2H-oxazolo[2,3-b]quinazoline-8-carboxylate synthesis
- Product Name:Methyl5-oxo-3,5-dihydro-2H-oxazolo[2,3-b]quinazoline-8-carboxylate
- CAS Number:1039454-98-2
- Molecular formula:C12H10N2O4
- Molecular Weight:246.22
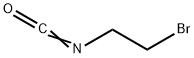
42865-19-0
29 suppliers
$91.00/1g
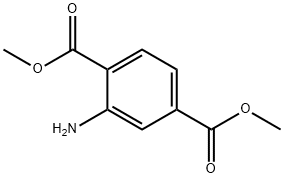
5372-81-6
229 suppliers
$17.67/5gm:
![Methyl5-oxo-3,5-dihydro-2H-oxazolo[2,3-b]quinazoline-8-carboxylate](/CAS2/GIF/1039454-98-2.gif)
1039454-98-2
5 suppliers
inquiry
Yield:1039454-98-2 73%
Reaction Conditions:
Stage #1: 2-bromoethyl isocyanate;dimethyl aminoterephthalate in 1,2-dichloro-ethane; for 3 h;Heating / reflux;
Stage #2: with triethylamine in 1,2-dichloro-ethane; for 24 h;Heating / reflux;
Steps:
60
Dimethyl aminoterephthalate (3.5 g, 14.5 mmol) and 2-bromoethyl isocyanate (3.5 g, 23.3 mmol) were dissolved in 1,2-dichloroethane (50 mL). The mixture was heated to reflux (90 0C) for three hours. Triethylamine (5 mL) was added and the mixture was heated at reflux for another 24 hours. The mixture was evaporated onto silica gel (10 g) which was transferred to a silica gel column (100 g) and eluted using chloroform:THF, 80:20. The product fractions were combined and concentrated under vacuum to give 2.6 g (73%) of an off white solid.The solid from the previous paragraph (1.56 g, 6.3 mmol) was dissolved in chloroform (150 mL). A mixture of nitric acid and sulfuric acid (1 : 1 , 23 mL) was added dropwise. During 2 hr of stirring, the solution temperature increased to 40 0C. The reaction was poured into ice/water and the product was extracted with chloroform. The organic phase was washed with a saturated sodium bicarbonate solution and dried over magnesium sulfate. The solution was concentrated under vacuum to give 1.63 g of white solid.The solid from the previous paragraph (1.63 g, 5.6 mmol) was dissolved in THF:MeOH (1:1, 100 mL) and the reduction of the nitro group (using freshly prepared Zn/Cu reagent) was carried out as in Example 3. The synthesis of the final product was carried out as in Example 2 substituting 3-fluorophenethylamine for 3-aminopropionitrile. The brown reaction mixture was diluted with chloroform (200 mL) and water (100 mL). The layers were separated and the
References:
WO2008/85505,2008,A1 Location in patent:Page/Page column 105-106