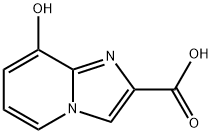
IMidazo[1,2-a]pyridine-2-carboxylic acid, 8-hydroxy- synthesis
- Product Name:IMidazo[1,2-a]pyridine-2-carboxylic acid, 8-hydroxy-
- CAS Number:1041004-62-9
- Molecular formula:C8H6N2O3
- Molecular Weight:178.14
![IMidazo[1,2-a]pyridine-2-carboxylic acid, 8-hydroxy-, ethyl ester](/CAS/GIF/1041004-63-0.gif)
1041004-63-0
21 suppliers
inquiry
![IMidazo[1,2-a]pyridine-2-carboxylic acid, 8-hydroxy-](/CAS/GIF/1041004-62-9.gif)
1041004-62-9
8 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: ethyl 8-hydroxyimidazo[1,2-a]pyridine-2-carboxylatewith water;sodium hydroxide in methanol at 20; for 3 h;
Stage #2: with hydrogenchloride in methanol; pH=2;
Steps:
8-Hydroxyimidazo[1,2-a]pyridine-2-carboxylic acid (7c)
To a suspension of ethyl 8-hydroxyimidazo[1,2-a]pyridine-2-carboxylate (300 mg, 1.45mmol, 1.0 eq.) in MeOH (10 mL), 1 M NaOH (aq. sol) (7.3 mL, 7.25 mmol, 5.0 eq.) was added. The reaction mixture was stirred at room temperature for 3 h, and then the organicsolvent was evaporated under reduced pressure. The remaining aqueous solution was acidifiedwith conc. HCl to pH 2, transferred into a separating funnel, and extracted with EtOAc (4× 30mL). The combined organic phases were dried over anhydrous Na2SO4, filtered, andevaporated under reduced pressure. The crude product was used in the next reaction stepwithout further purification. Brown solid, mp > 250 °C (decomposition); 1H NMR (400 MHz, DMSO-d6): δ 6.63(d, J = 7.2, 1.0 Hz, 1H), 6.78-6.81 (m, 1H), 8.08 (dd, J = 6.7, 0.9 Hz, 1H), 8.47 (s, 1H), 10.75(bs, 1H), resonance for COOH missing; ESI-HRMS m/z calcd. for C8H7N2O2 [M+H]+179.0457, found 179.0455.
References:
Knez, Damijan;Coquelle, Nicolas;Pi?lar, Anja;?akelj, Simon;Juki?, Marko;Sova, Matej;Mravljak, Janez;Nachon, Florian;Brazzolotto, Xavier;Kos, Janko;Colletier, Jacques-Philippe;Gobec, Stanislav [European Journal of Medicinal Chemistry,2018,vol. 156,p. 598 - 617] Location in patent:supporting information