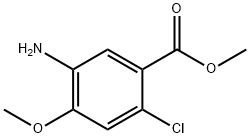
5-AMino-2-chloro-4-Methoxy-benzoic acid Methyl ester synthesis
- Product Name:5-AMino-2-chloro-4-Methoxy-benzoic acid Methyl ester
- CAS Number:104253-47-6
- Molecular formula:C9H10ClNO3
- Molecular Weight:215.63
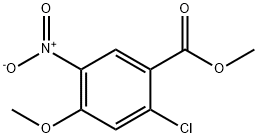
90537-46-5
19 suppliers
$220.00/1g

104253-47-6
16 suppliers
$45.00/10mg
Yield:104253-47-6 80%
Reaction Conditions:
with tin(ll) chloride in methanol at 65; for 2 h;
Steps:
90
Intermediate 90 : methyl 5-amino-2-chloro-4-methoxybenzoate; To a suspension of methyl 2-chloro-4-methoxy-5-nitrobenzoate (7.50 g, 30.5 mmol) in methanol (300 mL) was added tin(II) chloride (29.0 g, 152 mmol) in a single portion (reaction mixture turns yellow and gradually becomes homogeneous). The reaction mixture was heated to 65 0C for 2 h. The reaction mixture was evaporated and the residue diluted with water (300 mL). This was then neutralised with saturated aquoeus sodium bicarbonate (CAUTION: Froths - the froth could be dispersed by addition of a little EtOAc). To the resultant suspension was added EtOAc (300 mL). The mixture looked very difficult to separate and so was filtered to remove precipitated tin residues. The organic layer was separated, washed with brine, dried (Na2SO4) and evaporated to give methyl 5- amino-2-chloro-4-methoxybenzoate (5.30 g, 80 %) as a brown oil. This was used in the next step without purification, m/z (ESI+) (M+H)+ = 216; HPLC tR = 1.58 min.
References:
WO2009/47558,2009,A1 Location in patent:Page/Page column 164
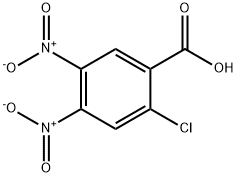
33458-98-9
4 suppliers
inquiry

104253-47-6
16 suppliers
$45.00/10mg