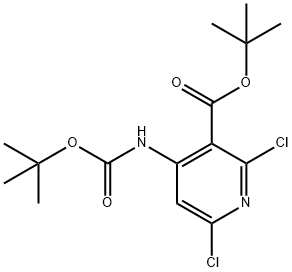
tert-butyl 4-(tert-butoxycarbonylamino)-2,6-dichloronicotinate synthesis
- Product Name:tert-butyl 4-(tert-butoxycarbonylamino)-2,6-dichloronicotinate
- CAS Number:1044148-92-6
- Molecular formula:C15H20Cl2N2O4
- Molecular Weight:363.24

1044148-88-0
22 suppliers
inquiry

1044148-92-6
11 suppliers
inquiry
Yield:1044148-92-6 100%
Reaction Conditions:
with lithium diisopropyl amide in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Steps:
2 Step 2-Tert-butyl 4-(tert-butoxycarbonylamino)-2,6-dichloro-pyridine-3-carboxylate
To a solution of LDA (2 M, 169 mL) was added a solution of tert-butyl N-tert-butoxycarbonyl-N-(2,6-dichloro-4-pyridyl)carbamate (35.0 g, 96.4 mmol) in THF (600 mL) under nitrogen at -78° C. The reaction mixture was stirred at -78° C. for 0.5 hr. On completion, the reaction mixture was quenched with saturated NH4Cl, and extracted with ethyl acetate (3×100 mL). The combined organic layer was washed with brine (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The residue was purified by silica gel chromatography (PE:EA=200:1) to give the title compound (35.0 g, 100% yield) as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.99 (s, 1H), 8.35 (s, 1H), 1.64 (s, 9H), 1.54 (s, 9H).
References:
US2019/192668,2019,A1 Location in patent:Paragraph 2642; 2644
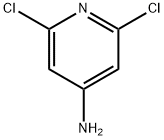
2587-02-2
293 suppliers
$12.40/250mg

24424-99-5
821 suppliers
$13.50/25G

1044148-92-6
11 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G

1044148-92-6
11 suppliers
inquiry

2587-02-2
293 suppliers
$12.40/250mg

1044148-92-6
11 suppliers
inquiry
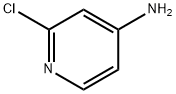
14432-12-3
522 suppliers
$5.00/5g

24424-99-5
821 suppliers
$13.50/25G

1044148-92-6
11 suppliers
inquiry