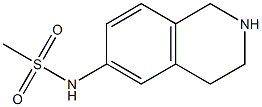
N-(1,2,3,4-tetrahydroisoquinolin-6-yl)methanesulfonamide synthesis
- Product Name:N-(1,2,3,4-tetrahydroisoquinolin-6-yl)methanesulfonamide
- CAS Number:1044766-32-6
- Molecular formula:C10H14N2O2S
- Molecular Weight:226.2954
![2(1H)-Isoquinolinecarboxylic acid, 3,4-dihydro-6-[(methylsulfonyl)amino]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/166398-32-9.gif)
166398-32-9
0 suppliers
inquiry

1044766-32-6
2 suppliers
inquiry
Yield:1044766-32-6 83%
Reaction Conditions:
with hydrogenchloride in dichloromethane;ethyl acetate at 20; for 8 h;
Steps:
22.4 Step 4) N- (1, 2, 3, 4-tetrahydroisoquinolin-6-yl) methanesulfonamide
Step 4) N- (1, 2, 3, 4-tetrahydroisoquinolin-6-yl) methanesulfonamide
To a solution of tert-butyl 6- (methylsulfonamido) -3, 4-dihydroisoquinoline-2 (1H) -carboxylate (326 mg, 1 mmol) in DCM (4 mL) was added an EtOAc solution of HCl (3 mL) . After the addition, the mixture was stirred at rt for 8 hours. The reaction mixture was concentrated in vacuo and the residue was suspended in EtOAc. The mixture was filtered to give light yellow solid, which was adjusted with aqueous NaHCO3to pH 8. The resulting mixture was filtered, and the filter cake washed with water and dried to give N- (1, 2, 3, 4-tetrahydroisoquinolin-6-yl) methanesulfonamide as a white solid (187 mg, 83) .[1076]MS (ESI, pos. ion) m/z: 227.1 [M+H]+.
References:
WO2015/197028,2015,A1 Location in patent:Paragraph 00133
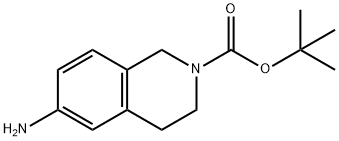
164148-92-9
148 suppliers
$11.00/100mg

1044766-32-6
2 suppliers
inquiry
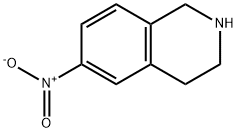
186390-77-2
53 suppliers
inquiry

1044766-32-6
2 suppliers
inquiry