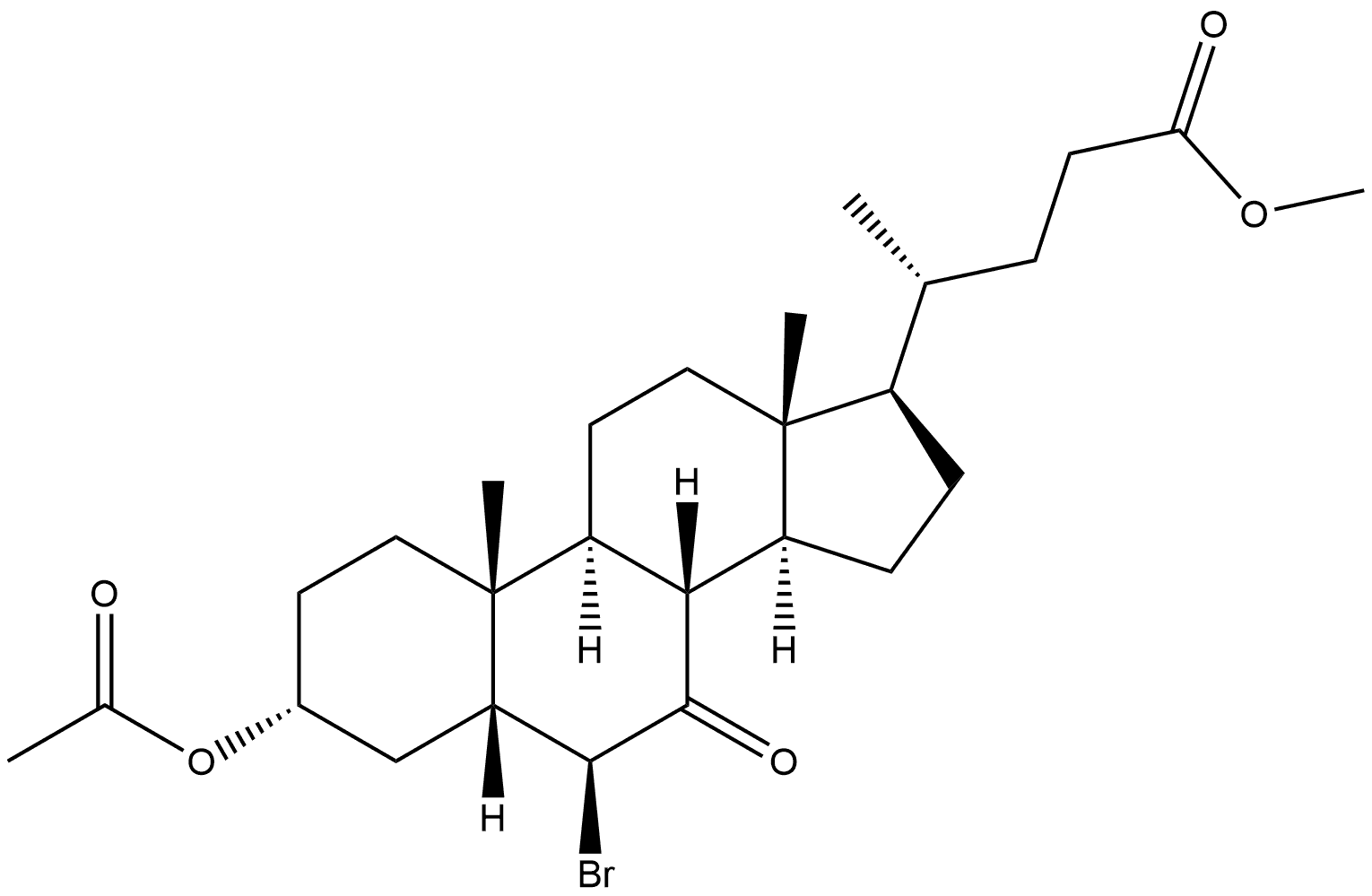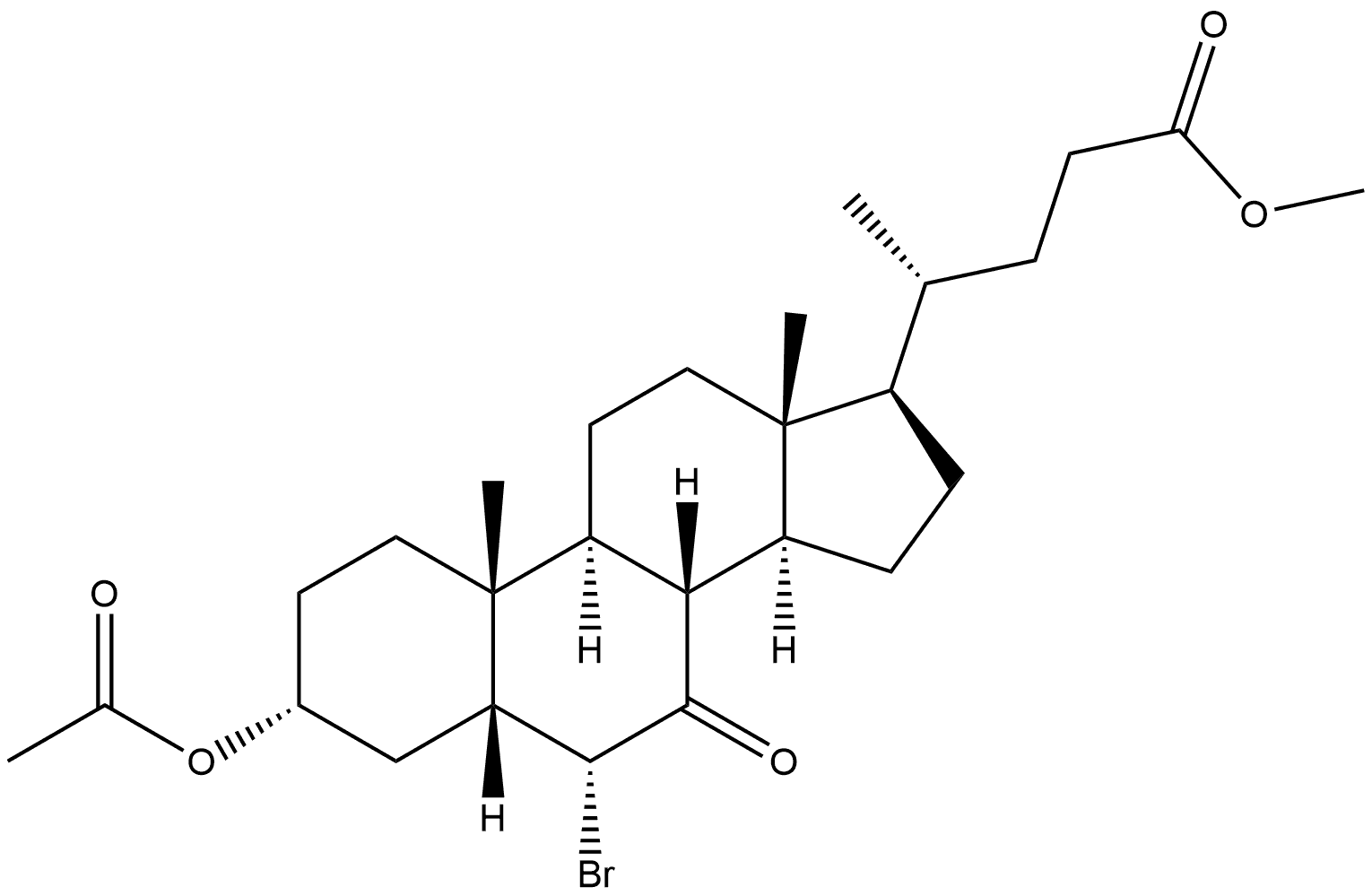
Methyl (3α,5β,6α)-3-(Acetyloxy)-6-bromo-7-oxo-cholan-24-oic Acid Ester synthesis
- Product Name:Methyl (3α,5β,6α)-3-(Acetyloxy)-6-bromo-7-oxo-cholan-24-oic Acid Ester
- CAS Number:10452-64-9
- Molecular formula:C27H41BrO5
- Molecular Weight:525.52
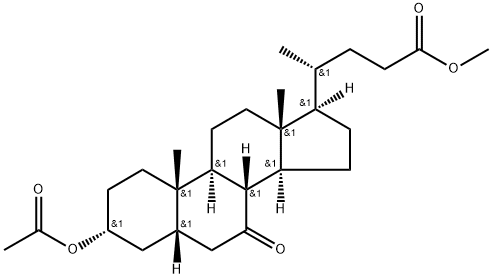
10452-65-0
5 suppliers
inquiry

10452-64-9
1 suppliers
inquiry
Yield:10452-64-9 76%
Reaction Conditions:
with hydrogen bromide;bromine;acetic acid in water at 20;
Steps:
2.2.3. Methyl 3α-acetoxy-6α-bromo-7-keto-5β-cholan-24-UDCA (3)
Bromine (1.5 mL, 0.5 mmol) was dissolved in acetic acid (21 mL) at0 °C and added dropwise to compound 2 (10.57 g, 23.7 mmol) at 0 °C.After finished, the solution of 48% HBr in water 1.1 mL was added intomixture. The reaction mixture was stirred at room temperature withTLC monitoring. After the reaction had finished, the mixture waspoured into NaHCO3 (aq), extracted with ethyl acetate 20 mL, thenwashed with Na2S2O3(aq), water and brine, dried with anhydrousNa2SO4 and concentrated. The residence was recrystallized by 10 mLMeOH at -20 °C, then filtered to give compound 3(9.433 g, 76%) as alight yellow solid. IR (ATR) cm-1: 3439, 2951, 2875, 2836, 1728. 1HNMR (300 MHz, CDCl3): 5.17 (1H, d, 6-H), 4.66 (1H, tt, 3-H), 3.62 (1H,s, 24-OCH3), 2.44 (1H, t, 8-H), 1.99 (3H, s, 3-Ac), 1.26 (3H, s, 18-H),0.88 (3H, d, 20-H), 0.64 (3H, s, 19-H); 13C NMR (75 MHz, CDCl3) δ201.28, 174.53, 170.30, 77.42, 77.00, 76.58, 72.37, 59.02, 54.68,53.89, 51.45, 49.46, 49.25, 42.96, 42.76, 38.60, 38.05, 35.07, 33.90,30.93, 30.85, 28.46, 28.09, 25.98, 24.40, 23.18, 21.68, 21.18, 18.28,11.98. HRMS (ESI): calcd for C27H41BrO5 [M+Na]+, 547.2035, found547.2034.
References:
Di, Xiangjie;Han, Jie;Huang, Qingfei;Wang, Qiwei;Wang, Yuanhua;Wei, Xia;Zhong, Liu;Zhu, Jin;Zou, Sheng [Steroids,2020,vol. 160]
4651-67-6
337 suppliers
$39.00/500mg

10452-64-9
1 suppliers
inquiry

10538-59-7
132 suppliers
inquiry

10452-64-9
1 suppliers
inquiry

474-25-9
686 suppliers
$20.80/5G

10452-64-9
1 suppliers
inquiry