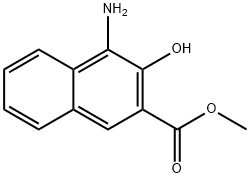
1-AMINO-2-HYDROXY-NAPHTALENE-3-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:1-AMINO-2-HYDROXY-NAPHTALENE-3-CARBOXYLIC ACID METHYL ESTER
- CAS Number:104655-33-6
- Molecular formula:C12H11NO3
- Molecular Weight:217.22
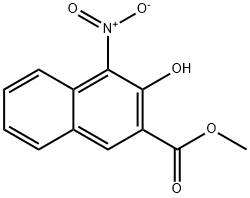
5394-81-0
12 suppliers
inquiry

104655-33-6
9 suppliers
inquiry
Yield:104655-33-6 37%
Reaction Conditions:
with iron;ammonium chloride in methanol;water; for 4 h;Reflux;
Steps:
Methyl 4-Amino-3-hydroxy-2-naphthoate (9): To a solution of 8 (99 mg, 0.4 mmol) in methanol-water(4 mL-1 mL) was added iron (112 mg, 2 mmol) and NH4Cl(170 mg, 3.2 mmol). The reaction mixture was heated under reflux for 4 h, then diluted with ethyl acetate (60 mL) andfiltered. The filtration was concentrated to give a residue thatwas purified by a silica gel column, eluting with hexanes:ethyl acetate:dichloromethane (4:1:1) to yield product 9(32 mg, 37%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ 10.49 (s, 1 H, OH), 7.99 (s, 1 H, Ar-H1), 7.79 (d, J = 8.3Hz, 1 H, Ar-H8), 7.74 (d, J = 8.6 Hz, 1 H, Ar-H5), 7.50 (td, J= 7.6, 1.3 Hz, 1 H, Ar-H6), 7.32 (t, J = 7. 6 Hz, 1 H, Ar-H7),4.27 (brs, 2 H, NH2), 4.02 (s, 3 H, OCH3).
References:
Xie, Fuchun;Li, Bingbing X.;Xiao, Xiangshu [Letters in Organic Chemistry,2013,vol. 10,# 5,p. 380 - 384]
883-99-8
107 suppliers
$20.00/5g

104655-33-6
9 suppliers
inquiry
288402-18-6
3 suppliers
inquiry

104655-33-6
9 suppliers
inquiry