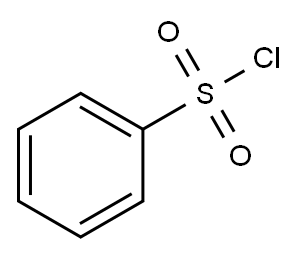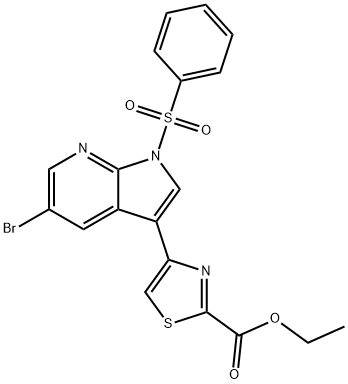
2-Thiazolecarboxylic acid, 4-[5-broMo-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]-, ethyl ester synthesis
- Product Name:2-Thiazolecarboxylic acid, 4-[5-broMo-1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]-, ethyl ester
- CAS Number:1046793-41-2
- Molecular formula:C19H14BrN3O4S2
- Molecular Weight:492.37
Yield:1046793-41-2 57%
Reaction Conditions:
with sodium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in dichloromethane;water at 20; for 1.5 h;
Steps:
4-(l-Benzenesulfonyl-5-bromo-lH-pyrrolo[2,3-b]pyridin-3-yl)-thiazole-2- carboxylic acid ethyl ester (III-a)(XXIII-a) ll-a)Compound (XXIII-a) (6.08 g, 14.0 mmol), W-Bu4NHSO4 (100 mg, cat.), PhSO2Cl (2.50 mL, 19.5 mmol) and 50% aqueous NaOH (2 mL) in CH2Cl2 (75 mL) were reacted at RT for 1.5 h following the general procedure for the protection of (XXIII). The crude product was recystallized from CH2Cl2/hexane to afford pure (III-a) (2.33 g) as a yellow solid. More product was obtained from the mother liquor by SGC using hexane:CH2Cl2:Et0Ac (2: 1 : 1, v/v/v) as eluent. Total yield of (III-a) (4.40 g, 8.94 mmol, 57%). 1U NMR (400 MHz, CDCl3) δ 1.51 (t, J = 7.2 Hz, 3H), 4.55 (q, J = 7.1 Hz, 2H), 7.53 (t, J = 7.8 Hz, 2H), 7.63 (t, J = 7.5 Hz, IH), 7.74 (s, IH), 8.24 (d, J = 7.8 Hz, I H), 8.30 (s, IH), 8.54 (d, J= 2.1 Hz, 1 H), 8.56 (d, J= 2.1 Hz, I H).; General procedure for protection of (XXIII)(XXIII) (III) To a stirred solution of (XXIII) (10 mmol) in CH2Cl2 (53 mL) was added n- Bu4NHSO4 (71 mg - 500 mg, 0.21 mmol - 1.47 mmol) and 50% aqueous NaOH (1.4 mL). PhSO2Cl (1.8 mL, 14 mmol) was then added dropwise, and the reaction stirred at RT for 1.5 h. The mixture was then diluted with EtOAc (290 mL) and acetone (14 mL), washed with brine (2 x 70 mL) and concentrated to give crude (III). This product can be purified by SGC using appropriate solvent system as eluent or recrystallized from CH2Cl2/hexane to
References:
WO2008/95943,2008,A1 Location in patent:Page/Page column 54-55; 164
![2-Thiazolecarboxylic acid, 4-(5-broMo-1H-pyrrolo[2,3-b]pyridin-3-yl)-, ethyl ester](/CAS/GIF/1046793-71-8.gif)