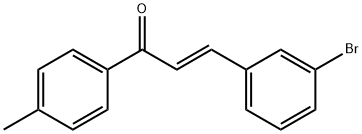
(2E)-3-(3-bromophenyl)-1-(4-methylphenyl)prop-2-en-1-one synthesis
- Product Name:(2E)-3-(3-bromophenyl)-1-(4-methylphenyl)prop-2-en-1-one
- CAS Number:1047680-88-5
- Molecular formula:C16H13BrO
- Molecular Weight:301.18

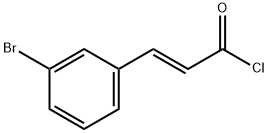
2039-86-3
154 suppliers
$15.00/1g
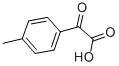
7163-50-0
37 suppliers
$110.00/1g

1047680-88-5
4 suppliers
inquiry
Yield:1047680-88-5 71%
Reaction Conditions:
with dipotassium peroxodisulfate;silver carbonate in acetonitrile at 100; for 24 h;Schlenk technique;Inert atmosphere;Glovebox;
Steps:
General procedure for the decarboxylative cross-coupling of α-keto acids with alkenes
General procedure: 2-oxo-2-phenylacetic acid (1a, 0.2 mmol, 30.0 mg), K2S2O8 (0.6 mmol, 162.2 mg), Ag2CO3 (5.5 mg, 0.02 mmol) were added to the Schlenk tube equipped with a magnetic stir bar in an argon-filled glove box. 1-methyl-4-vinylbenzene (2a, 1.0 mmol, 104.2 mg), MeCN (1.0 mL) were injected into the reaction tube. The reaction was then heated up to 100 °C and stirred for 24 h. Upon completion, the reaction was quenched with water and extracted with ethyl ether (3 × 10 mL). The organic layers were then combined. The pure product 3a was obtained by flash column chromatography on silica gel (petroleum/ethyl acetate= 200:1).
References:
Wu, Shang;Yu, Hongheng;Hu, Qinzheng;Yang, Quanlu;Xu, Shouwang;Liu, Tian [Tetrahedron Letters,2017,vol. 58,# 51,p. 4763 - 4765] Location in patent:supporting information
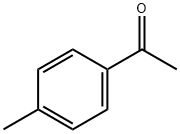
122-00-9
681 suppliers
$5.00/25g

1047680-88-5
4 suppliers
inquiry