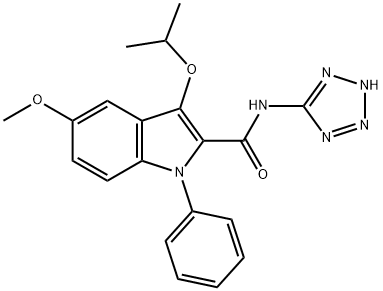
1H-Indole-2-carboxamide, 5-methoxy-3-(1-methylethoxy)-1-phenyl-N-2H-tetrazol-5-yl- synthesis
- Product Name:1H-Indole-2-carboxamide, 5-methoxy-3-(1-methylethoxy)-1-phenyl-N-2H-tetrazol-5-yl-
- CAS Number:104961-19-5
- Molecular formula:C20H20N6O3
- Molecular Weight:392.4112
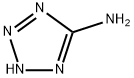
4418-61-5
452 suppliers
$10.00/5g
105012-91-7
2 suppliers
inquiry
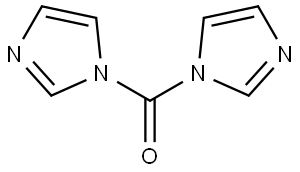
530-62-1
926 suppliers
$6.00/25g

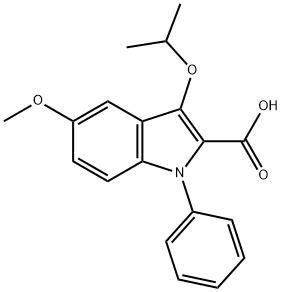
104961-19-5
5 suppliers
inquiry
Yield:104961-19-5 79%
Reaction Conditions:
with triethylamine in acetonitrile;
Steps:
25 5-Methoxy-3-(1-methylethoxy)-1-phenyl-N-1H-tetrazol-5-yl-1H-indole-2-carboxamide
EXAMPLE 25 (PROCEDURE B) 5-Methoxy-3-(1-methylethoxy)-1-phenyl-N-1H-tetrazol-5-yl-1H-indole-2-carboxamide A mixture of 20.0 g (0.062 mole) of 5-methoxy-3-(1-methylethoxy)-1-phenyl-1H-indole-2-carboxylic acid and 11.3 g (0.070 mole) of 1,1'-carbonyldiimidazole in 375 ml of acetonitrile was stirred at reflux (under a nitrogen atmosphere) for 90 minutes. The mixture was cooled, and 6.2 g (0.073 mole) of anhydrous 5-aminotetrazole was added, followed by 20.6 ml (15 g; 0.15 mole) of triethylamine. After stirring at reflux for an additional 16 hours, the mixture was cooled, added to 1.5 kg of ice/water and acidified with glacial acetic acid. The precipitated product was filtered and washed with water. Recrystallization from aqueous acetonitrile yielded 19.0 g (79% yield) of analytically pure carbamoyltetrazole product, mp 227° C.-dec.
References:
US4675332,1987,A