
Ethyl 3-broMo-2-(broMoMethyl)-4,6-diMethoxybenzoate synthesis
- Product Name:Ethyl 3-broMo-2-(broMoMethyl)-4,6-diMethoxybenzoate
- CAS Number:105208-04-6
- Molecular formula:C12H14Br2O4
- Molecular Weight:382.05

57749-86-7
6 suppliers
$68.09/100mg

105208-04-6
3 suppliers
inquiry
Yield:105208-04-6 44.1%
Reaction Conditions:
Stage #1: ethyl 2,4-dimethoxy-6-methylbenzoatewith N-Bromosuccinimide in Carbon tetrachloride at 80; for 1 h;Inert atmosphere;
Stage #2: with N-Bromosuccinimide;dibenzoyl peroxide in Carbon tetrachloride at 80; for 3 h;Inert atmosphere;
Steps:
Ethyl 3-bromo-2-(bromomethyl)-4,6-dimethoxybenzoate (12)
Following a modified literature procedure[3], ethyl 2,4-dimethoxy-6-methylbenzoate (2.0 g, 8.9 mmol) and N-bromosuccinimide (NBS, 1.7 g, 9.5 mmol) were dissolved in carbon tetrachloride (10 mL). The reaction mixture was refluxed for 1 h at 80 °C under nitrogen atmosphere. Additional NBS (3.8 g, 21.3 mmol) and benzoyl peroxide (BPO, 3.2 g, 13.2 mmol) were added. After refluxing for 3 h at 80 , the mixture was filtered and concentrated in vacuo to give an orange solid. The crude product was purified by column chromatography over silica gel (petroleum ether: ethyl acetate = 20:3, v/v) to afford the title compound (1.5 g, 44.1%) as a yellow solid. 1H NMR (400 MHz, CDCl3, ppm) δ 6.45 (s, 1H), 4.64 (s, 2H), 4.41 (q, J = 7.1 Hz, 2H), 3.91 (s, 3H), 3.84 (s, 3H), 1.39 (t, J = 7.1 Hz, 3H); HRMS (ESI): m/z [M+H]+ calcd for [C12H15Br2O4] +: 380.9337, found 380.9335
References:
Guo, Bing;Hu, Chu-Jiao;Peng, Jin-Gang;Tang, Lei;Wang, Jian-Ta;Xia, Jing;Zhang, Ji-Quan;Zhu, Gao-Feng [Bioorganic and Medicinal Chemistry Letters,2021,vol. 52,art. no. 128410] Location in patent:supporting information
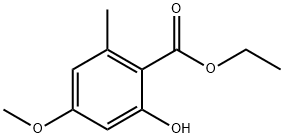
6110-36-7
6 suppliers
inquiry

105208-04-6
3 suppliers
inquiry