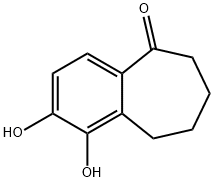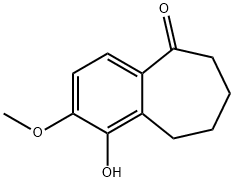
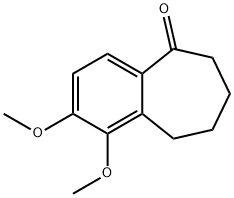
1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE synthesis
- Product Name:1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE
- CAS Number:105208-21-7
- Molecular formula:C12H14O3
- Molecular Weight:206.24
Yield:105208-21-7 77% ,54131-01-0 9%
Reaction Conditions:
Stage #1: 2,3-dimethoxy-6,7,8,9-tetrahydro-5H-benzo[a]cyclohepten-5-onewith trimethylammonium heptachlorodialuminate in dichloromethane at 80; for 1 h;Microwave irradiation;Inert atmosphere;
Stage #2: with water in dichloromethane;
Steps:
A.20
A solution of ketone 19 (2.22 g, 10.1 mmol) in CH2Cl2 (5 mL) and ionic liquid (13.00 mL, 1.93M [TMAH][Al2Cl7] soln. in CH2Cl2) was heated to 80° C. in a microwave for 1 h. After the reaction was complete water was added to quench the reaction. The mixture was stirred vigorously for 2 min and the organic layer was separated. Aqueous layer was extracted with CH2Cl2 (2*25 mL). Combined organic phase was washed with brine, and dried over MgSO4, filtered and evaporated under reduced pressure. Flash chromatography of the crude using a prepacked 100 g silica column, Eluents; solvent A, EtOAc, solvent B, hexanes; gradient, 10% A/90% B over 3.18 min (1 CV), 10% A/90% B→50% A/50% B over 33.0 min (10 CV), 50% A/50% B over 6.36 min (2 CV); flow rate 40.0 mL/min; monitored at λλ254 and 280 nm; afforded phenol 20 (1.59 g, 7.70 mmol, 77%) and catechol 59 (0.18 g, 0.94 mmol, 9%). 1H NMR (CDCl3, 500 MHz) (phenol 20): δ 7.34 (1H, d, J=8.6 Hz, H-4), 6.79 (1H, d, J=8.6 Hz, H-3), 3.94 (3H, s, OCH3-2), 3.02 (3H, s, CH2-5), 2.71 (2H, t, J=12.0 Hz, H-8), 1.85 (2H, s, CH2-6), 1.80 (2H, s, CH2-7). 13C NMR (CDCl3, 125 MHz): δ 205.0 (C, C-5), 149.2 (C, C-2), 142.4 (C, C-1), 133.3 (CH, C-4-a), 127.7 (C, C-1a), 120.8 (C, C-4), 107.9 (C, C-3), 56.1 (CH3, OCH3-2), 40.8 (CH, C-6), 24.5 (CH, C-8), 23.1 (CH, C-9), 21.3 (CH, C-7). Anal., Calcd for C12H14O3: C, 69.88; H, 6.84; 0, 23.27. Found: C, 69.93; H, 6.86. HRMS, m/z: observed 207.1016 [M+1]+, (calcd for C12H15O3+, 207.1016).
References:
US2012/130129,2012,A1 Location in patent:Page/Page column 10
54130-94-8
6 suppliers
inquiry
![1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE](/CAS/20180808/GIF/105208-21-7.gif)
105208-21-7
5 suppliers
inquiry
86-51-1
349 suppliers
$6.00/5g
![1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE](/CAS/20180808/GIF/105208-21-7.gif)
105208-21-7
5 suppliers
inquiry
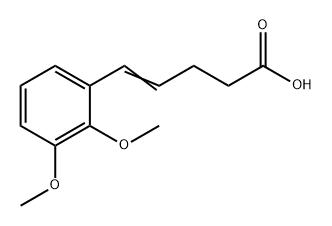
1378238-71-1
0 suppliers
inquiry
![1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE](/CAS/20180808/GIF/105208-21-7.gif)
105208-21-7
5 suppliers
inquiry
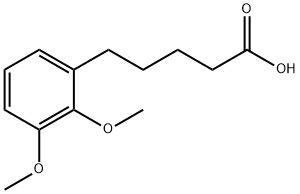
54130-93-7
4 suppliers
inquiry
![1-HYDROXY-2-METHOXY-6,7,8,9-TETRAHYDRO-5H-BENZO[7]ANNULEN-5-ONE](/CAS/20180808/GIF/105208-21-7.gif)
105208-21-7
5 suppliers
inquiry