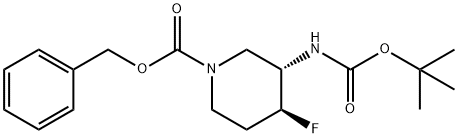
tert-butyl ((3S,4S)-1-benzyl-4-fluoropiperidin-3-yl)carbamate synthesis
- Product Name:tert-butyl ((3S,4S)-1-benzyl-4-fluoropiperidin-3-yl)carbamate
- CAS Number:1052713-39-9
- Molecular formula:C18H25FN2O4
- Molecular Weight:352.4
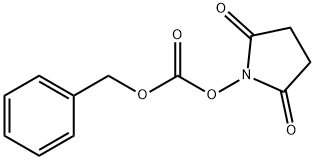
13139-17-8
502 suppliers
$6.00/25g
![tert-butyl N-[(3S,4S)-4-fluoropiperidin-3-yl]carbamate](/CAS/GIF/1052713-48-0.gif)
1052713-48-0
44 suppliers
$75.00/1g

1052713-39-9
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20; for 24 h;
Steps:
4.1 Step 1.
Triethylamine (0.19 mL, 1.37 mmol) was added to a solution of tert-butyl N-[(3S,4S)-4- fluoro-3-piperidyl]carbamate (250 mg, 1.15 mmol) in tetrahydrofuran (3 mL), followed by addition of N-(Benzyloxycabronyloxy) succinimide (430 mg, 1.73 mmol). The resulting reaction mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with ethyl acetate and quenched with saturated ammonium chloride solution, extracting with ethyl acetate twice. The combined organics were dried over magnesium sulfate and concentrated to produce the crude product, which was purified via silica gel column chromatography (0-40% ethyl acetate in hexanes) to yield benzyl (3S,4S)-3-(tert-butoxycarbonylamino)-4-fluoro-piperidine-1- carboxylate. ES/MS: m/z 353.74 [M+H]+.
References:
WO2021/222353,2021,A1 Location in patent:Page/Page column 377; 396

24424-99-5
820 suppliers
$13.50/25G
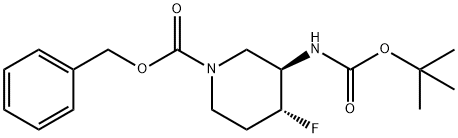
1052713-38-8
10 suppliers
inquiry

1052713-39-9
7 suppliers
inquiry
66207-08-7
89 suppliers
$46.00/1g

1052713-38-8
10 suppliers
inquiry

1052713-39-9
7 suppliers
inquiry
![1-Piperidinecarboxylic acid, 3-[[(1,1-dimethylethoxy)carbonyl]amino]-4-fluoro-, phenylmethyl ester](/CAS/20210305/GIF/1214242-81-5.gif)