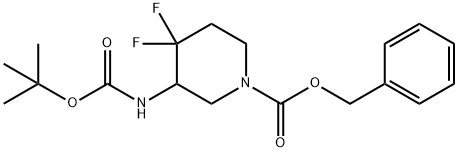
benzyl 3-((tert-butoxycarbonyl)amino)-4,4-difluoropiperidine-1-carboxylate(WX191889) synthesis
- Product Name:benzyl 3-((tert-butoxycarbonyl)amino)-4,4-difluoropiperidine-1-carboxylate(WX191889)
- CAS Number:1052713-44-6
- Molecular formula:C18H24F2N2O4
- Molecular Weight:370.39
![1,4-Dithia-8-azaspiro[4.5]decane-8-carboxylic acid, 6-[[(1,1-dimethylethoxy)carbonyl]amino]-, phenylmethyl ester](/CAS/20210305/GIF/189947-48-6.gif)
189947-48-6
0 suppliers
inquiry

1052713-44-6
11 suppliers
inquiry
Yield:1052713-44-6 12.5%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;pyridine hydrofluoride in dichloromethane at -78;
Steps:
11
Preparation of compound 11f: benzyl 3-[(terf-butoxycarbonyl)amino]-4,4- difluoropiperidine-1-carboxylate1 ,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (4.8 g, 17 mmol) was dissolved in dry CH2Cb (100 ml_) and allowed to stir under nitrogen. The mixture was cooled to - 78 0C and pyhdinium poly(hydrogen fluoride) (13 ml_) was added via polyethylene syringe, followed by the dropwise addition of compound 11e (6.5 g, 15.3 mmol). After 10 min, the reaction mixture, which was deep red, was diluted with hexane (1 L) and filtered through a column of basic alumina. The organic was washed with NaHCO3, brine, concentrated and purified by column chromatography (Petroleum Ether: EtOAc = 50:1 ) which gave the title compound 11f as a colorless oil (1.2 g, 12.5%). 1 H NMR (400 MHz, CDCI3) 1.380 (s, 9H), 2.133-1.874 (m, 2H), 3.159-3.101 (m, 1 H), 4.078- 3.927 (m, 3H), 4.687-4.670 (d, 1 H), 5.274-5.030 (m, 2H), 7.300-7.210 (m, 5H).
References:
WO2010/16005,2010,A1 Location in patent:Page/Page column 97