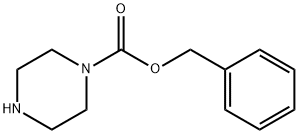
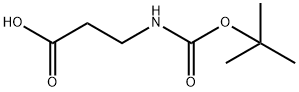
Carbamic acid, N-[3-oxo-3-(1-piperazinyl)propyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[3-oxo-3-(1-piperazinyl)propyl]-, 1,1-dimethylethyl ester
- CAS Number:1052715-64-6
- Molecular formula:C12H23N3O3
- Molecular Weight:257.33
Yield:1052715-64-6 60%
Reaction Conditions:
Stage #1: 3-(tert-butyloxycarbonylamino)propionic acidwith 1,1'-carbonyldiimidazole in N,N-dimethyl-formamide at 20; for 2 h;
Stage #2: phenylmethyl 1-piperazinecarboxylate in N,N-dimethyl-formamide; for 17 h;
Steps:
1.1 1) Synthesis of tert-butyl (3-oxo-3- (piperazin-1-yl) propyl) carbamate:
Dissolve 3-((tert-butoxycarbonyl) amino) propanoic acid (Boc protected amino acid, 7.6 g, 40 mmol, 1.0 eq) in DMF (100 mL), and add CDI (6.5 g, 40 mmol, 1.0 eq) at room temperature Stir for 2h, Then benzylpiperazine-1-carboxylic acid ester (10.6 g, 48 mmol, 1.2 eq) was added and the reaction was stirred for 17 h. The organic layer was separated and washed with saturated NaHCO3 solution (2 × 150 mL), 1N HCl solution (150 mL) and brine (150 mL), EA extraction, dried over anhydrous sodium sulfate, most of the solvent was removed by spin chromatography, and purified by silica gel column chromatography. Elution with petroleum ether / ethyl acetate (V / V = 20/1) gave the first intermediate (9.4 g) in a yield of 60%.
References:
CN110845445,2020,A Location in patent:Paragraph 0093-0097