5-(2-Amino-1,3-thiazol-4-yl)-2,3-dihydro-1H-indol-2-one synthesis
- Product Name:5-(2-Amino-1,3-thiazol-4-yl)-2,3-dihydro-1H-indol-2-one
- CAS Number:105316-99-2
- Molecular formula:C11H9N3OS
- Molecular Weight:231.27
Yield:105316-99-2 92%
Reaction Conditions:
in ethanol at 120; for 0.583333 h;Sealed tube;Inert atmosphere;Microwave irradiation;
Steps:
Intermediate 3
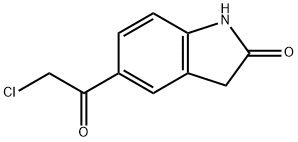
5-Chloroacetyloxidole (2.00 g, 9.54 mmol) and thiourea (0.76 g, 10.02 mmol) were suspended in anhydrous ethanol (15 ml) in a sealed tube under argon atmosphere.
The sealed tube was microwaved at 120° C. for 35 min.
The mixture diluted with tetrahydrofuran (200 mL) and neutralized with 1N aq. NaOH solution and then extracted with ethyl acetate (2*200 mL).
The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo.
The residue was purified by precipitating in dichloromethane and filtration to give 5-(2-aminothiazol-4-yl)indolin-2-one (2.36 g, 92%) as a light brown solid. 1H NMR (400 MHz, DMSO-d): δ 10.64 (s, 1H), 8.95 (bs, 1H), 7.62 (s, 1H), 7.60 (dd, 1H, J=8.4, 1.2 Hz), 7.06 (s, 1H), 6.89 (d, 1H, J=8.4 Hz). MS (ESI): Calcd. for C11H10ClN3OS: 267, found 268 (M+1)+.
References:
US2018/86752,2018,A1 Location in patent:Paragraph 0060; 0061

105316-98-1
27 suppliers
$45.00/10mg

105316-99-2
6 suppliers
inquiry
59-48-3
459 suppliers
$5.00/1g

105316-99-2
6 suppliers
inquiry