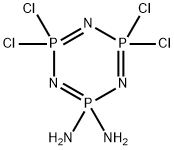
4,4,6,6-tetrachloro-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyc lohexa-1,3,5-triene-2,2-diamine synthesis
- Product Name:4,4,6,6-tetrachloro-1,3,5-triaza-2$l^{5},4$l^{5},6$l^{5}-triphosphacyc lohexa-1,3,5-triene-2,2-diamine
- CAS Number:10535-05-4
- Molecular formula:Cl4H4N5P3
- Molecular Weight:308.8
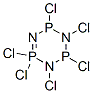
940-71-6
337 suppliers
$20.00/5G
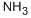
7664-41-7
491 suppliers
$15.00/100ml

10535-05-4
3 suppliers
inquiry
Yield:10535-05-4 95%
Reaction Conditions:
in diethyl ether at 0; for 1.5 h;
Steps:
Reaction of HCCP (1) and gaseous ammonia
General procedure: A typical procedure is as follows. Into a 100 mLtwo-necked flask were placed HCCP (1, 1392.4 mg, 4.0 mmol), MeCN (25 mL), and a stirrer bar. Thereaction vessel was purged with He, and NH3 (1283 mL, 51 mmol) was introduced by using a syringe anda syringe pump for 30 min. The reaction mixture was stirred for 1 h to form colorless precipitates. Thereaction mixture was filtered, and the precipitates were washed with hot MeCN. The filtrate wasconcentrated under reduced pressure to give colorless solids. The solids were dispersed in Et2O, filtered,and washed with Et2O to give 2,2-diamino-4,4,6,6-tetrachlorocyclotriphosphazene (2, filtrate, 14.4 mg,0.05 mmol, 1%) and 2,2,4,4-tetraamino-6,6-dichlorocyclotriphosphazene (4, precipitates, 978.8 mg, 3.63mmol, 91%). The filtrate only contained 2 and the precipitates only contained 4. 2: 1H NMR (399.3 MHz, CDCl3) ? 2.00 (s, 4H), 31P NMR (161.6 MHz, MeCN (D2O))12 .. 20.0 (d, J = 49.4 Hz, 2P), 10.8 (t,J = 49.5 Hz, 1P). 4: 1H NMR (399.3 MHz, CD3CN) .. 1.92 (s, 8H), 31P NMR (161.6 MHz, MeCN(D2O))12 .. 22.6 (t, J = 53.2 Hz, 1P), 13.8 (d, J = 53.2 Hz, 2P).
References:
Kuroboshi, Manabu;Morita, Masahiko;Masumoto, Yasunari;Mikasa, Masaki;Toza, Ryohei;Tanaka, Hideo [Heterocycles,2019,vol. 98,# 7,p. 931 - 939]