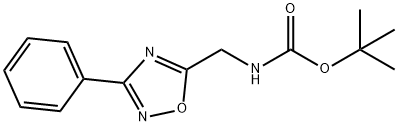
5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole synthesis
- Product Name:5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole
- CAS Number:1053656-50-0
- Molecular formula:C14H17N3O3
- Molecular Weight:275.3
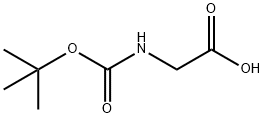
![Glycine, N-[(1,1-dimethylethoxy)carbonyl]-, 1,1'-anhydride](/CAS/20211123/GIF/51499-90-2.gif)
51499-90-2
2 suppliers
inquiry
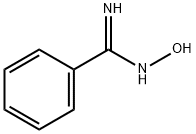
613-92-3
197 suppliers
$5.00/250mg
![5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole](/CAS/GIF/1053656-50-0.gif)
1053656-50-0
9 suppliers
$81.59/500mg
Yield:1053656-50-0 91%
Reaction Conditions:
in neat (no solvent) at 80; for 0.116667 h;Green chemistry;
Steps:
tert-Butyl (3-phenyl-1,2,4-oxadiazol-5-yl)methyl carbamate (Table 2, 5m):
A mixture of benzamidoxime (1.47 mmol), compound 4h (1.77 mmol) and HClO4-SiO2 (5 mol%) was stirred at 80 °C for 7 min. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature, diluted with dichloromethane (5 mL) and the catalyst was allowed to settle down. Then the reaction mixture was filtered, washed with dichloromethane (5 mL), and the combined organic layers were washed with saturated aq. NaHCO3 and water. The obtained organic layer was dried over anhy. Na2SO4 and concentrated under reduced pressure to get crude compound. The crude was then purified by column chromatography on silica gel using hexane/EtOAc (8:2) as eluents to get pure compound 5m.
References:
Tadikonda, Ramu;Nakka, Mangarao;Gajula, Mahaboob Basha;Rayavarapu, Srinuvasarao;Gollamudi, Padma Rao;Vidavalur, Siddaiah [Synthetic Communications,2014,vol. 44,# 13,p. 1978 - 1986] Location in patent:supporting information
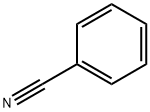
4530-20-5
531 suppliers
$5.00/5G

613-92-3
197 suppliers
$5.00/250mg
![5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole](/CAS/GIF/1053656-50-0.gif)
1053656-50-0
9 suppliers
$81.59/500mg
100-47-0
359 suppliers
$14.14/25gm:
![5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole](/CAS/GIF/1053656-50-0.gif)
1053656-50-0
9 suppliers
$81.59/500mg

4530-20-5
531 suppliers
$5.00/5G
![5-(tert-Butyloxycarbonyamino)methyl-3-phenyl-[1,2,4]oxadiazole](/CAS/GIF/1053656-50-0.gif)
1053656-50-0
9 suppliers
$81.59/500mg