
1,2-di-[4-(aminomethyl)phenyl]-1,2-diphenylethylene synthesis
- Product Name:1,2-di-[4-(aminomethyl)phenyl]-1,2-diphenylethylene
- CAS Number:1054451-32-9
- Molecular formula:C28H26N2
- Molecular Weight:390.52
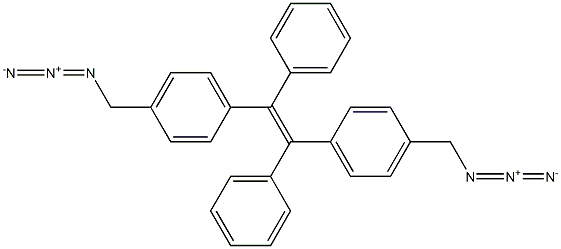
1054451-33-0
9 suppliers
$518.00/25mg
![1,2-di-[4-(aminomethyl)phenyl]-1,2-diphenylethylene](/CAS/20200119/GIF/1054451-32-9.gif)
1054451-32-9
17 suppliers
inquiry
Yield:1054451-32-9 87%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 8 h;Inert atmosphere;Reflux;
Steps:
15 1,2-di[4-(aminomethyl)phenyl]-1,2-diphenylethylene (TPE-MA)
The azido-substituted TPE (TPE-MN3) (0.3 g, 0.7 mmol) was dissolved in dry THF (60 ml) and LiAlH4 (0.15 g, 4.1 mmol) was added slowly at room temperature with constant stirring under nitrogen. Following the addition, the mixture was heated at reflux for 8 h. Water (5 ml-10 ml) was added slowly to decompose the excess LiAlH4. The solution was then filtered and THF was used to wash the solid residue. After evaporation of the organic filtrate, dilute hydrochloric acid was added and the aqueous solution was washed by diethyl ether for several times. Ammonium hydroxide was used to basify the aqueous solution, following the extraction by diethyl ether. The organic layer was dried with Na2SO4 and evaporated to dryness. TPE-MA was obtained as light yellow powder in 87% yield. TPE-MA: 1H NMR (d-MeOH, 300 MHz) δ (ppm): 7.10-7.06 (m, 10H), 7.00-6.93 (m, 8H), 3.75-3.70 (hr, 4H); 13C NMR (CDCl3, 75 MHz), δ (TMS, ppm): 143.9, 142.4, 140.7, 131.6, 131.4, 127.8, 127.7, 126.5, 126.4, 30.4; MS (TOF) m/e: 374.1 ([M-NH2]+ calcd: 374.2)
References:
US9279806,2016,B2 Location in patent:Page/Page column 64; 65
![1,2-Bis[4-(bromomethyl)phenyl]-1,2-diphenylethene](/CAS/20180703/GIF/1053241-67-0.gif)
1053241-67-0
48 suppliers
$518.00/25mg
![1,2-di-[4-(aminomethyl)phenyl]-1,2-diphenylethylene](/CAS/20200119/GIF/1054451-32-9.gif)
1054451-32-9
17 suppliers
inquiry
134-84-9
459 suppliers
$7.00/10g
![1,2-di-[4-(aminomethyl)phenyl]-1,2-diphenylethylene](/CAS/20200119/GIF/1054451-32-9.gif)
1054451-32-9
17 suppliers
inquiry