
2-(2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL)ETHANAMINE HYDROCHLORIDE synthesis
- Product Name:2-(2,3-DIHYDRO-1,4-BENZODIOXIN-6-YL)ETHANAMINE HYDROCHLORIDE
- CAS Number:10554-64-0
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
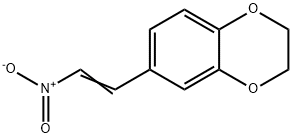
10554-65-1
16 suppliers
inquiry

10554-64-0
17 suppliers
$200.00/50mg
Yield:10554-64-0 61%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20;Reagent/catalyst;Solvent;
Steps:
5.1.3 2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)ethylamine (3)
(E)-6-(2-Nitrovinyl)-2,3-dihydro[1,4]benzodioxine (2) (1g, 4.82mmol) was dissolved in 102 THF (20mL). 36 LiAlH4 (740mg, 19.5mmol) was added portion wise. The reaction was stirred at rt for 20h and TLC of the reaction mixture (hexane/EtOAc 1:1) indicated formation of a new compound and complete consumption of starting material (Rf=0.60). The crude mixture was quenched dropwise with 103 water (1mL) and filtered. The solid residue was extracted with CH2Cl2 (3×20mL) and the combined organic phases were dried (Na2SO4), filtered and concentrated in vacuo to afford 17 2-(2,3-dihydro[1,4]benzodioxin-6-yl)ethylamine (3) (530mg, 61% yield) as a pale brown oil. This material was identical in all respects with that previously described.
References:
Capilla, A. Sergi;Soucek, Richard;Grau, Laura;Romero, Manel;Rubio-Martínez, Jaime;Caignard, Daniel H.;Pujol, Maria Dolors [European Journal of Medicinal Chemistry,2018,vol. 145,p. 51 - 63]
17253-10-0
10 suppliers
inquiry

10554-64-0
17 suppliers
$200.00/50mg
29668-44-8
269 suppliers
$6.00/1g

10554-64-0
17 suppliers
$200.00/50mg
139-85-5
812 suppliers
$5.00/1g

10554-64-0
17 suppliers
$200.00/50mg
858789-85-2
2 suppliers
inquiry

10554-64-0
17 suppliers
$200.00/50mg