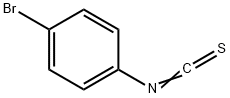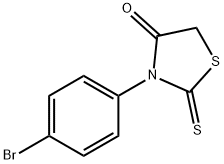
3-(4-bromophenyl)-2-sulfanylidene-thiazolidin-4-one synthesis
- Product Name:3-(4-bromophenyl)-2-sulfanylidene-thiazolidin-4-one
- CAS Number:10574-70-6
- Molecular formula:C9H6BrNOS2
- Molecular Weight:288.18
Yield:10574-70-6 45%
Reaction Conditions:
in water at 95 - 100; for 19 h;
Steps:
5.1.9. General procedure for preparation of 3-aryl-2-thioxothiazolidin-4-one 12a-d and 12f-j
General procedure: A suspension of aniline analogues (1 equiv) in water (ca. 2 mL/mmol) was heated to 95 °C until the aniline was fully dissolved. Then bis(carboxylmethyl) trithiocarbonate (1.1 equiv) was added and heated at 100 °C for 19 h. After cooling to room temperature, the precipitated solid was filtered out and washed with water. The dried solid was purified by flash silica column [gradual eluent: EtOAc/petroleum ether, 0-50%] to afford the rhodanine derivatives.
References:
He, Xiao-Yang;Zou, Peng;Qiu, Jiayin;Hou, Ling;Jiang, Shibo;Liu, Shuwen;Xie, Lan [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 22,p. 6726 - 6734] Location in patent:experimental part