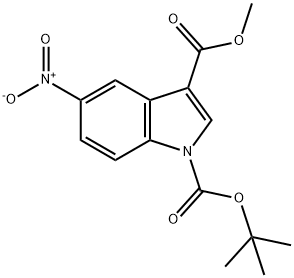
1-TERT-BUTYL 3-METHYL 5-NITRO-1H-INDOLE-1,3-DICARBOXYLATE synthesis
- Product Name:1-TERT-BUTYL 3-METHYL 5-NITRO-1H-INDOLE-1,3-DICARBOXYLATE
- CAS Number:1058740-09-2
- Molecular formula:C15H16N2O6
- Molecular Weight:320.3

686747-51-3
57 suppliers
$54.00/250mg

24424-99-5
840 suppliers
$13.50/25G

1058740-09-2
10 suppliers
inquiry
Yield:1058740-09-2 86%
Reaction Conditions:
with dmap in tetrahydrofuran;acetonitrile at 20 - 30;
Steps:
L
Example L; 1 -tert-butyl-3-methyl 5-nitro-indole-1 ,3-dicarboxylate; 5.9 g methyl 5-nitro-1 H-indole-3-carboxylate are dissolved in 100 ml acetonitrile, combined with 100 mg N,N-dimethylamino-pyhdine and a solution of 6.4 g di-tert.-butyl-dicarbonate in 20 ml of tetrahydrofuran is added dropwise thereto. The mixture is stirred overnight at ambient temperature, then heated to 300C for 30 minutes and cooled to 00C. The solid thus precipitated is suction filtered. The mother liquor is freed from the solvents in vacuo and the residue is extracted from petroleum ether/ethyl acetate. Yield: 7.4 g (86 % of theory) Rf value: 0.50 (silica gel: petroleum ether/ethyl acetate 3:1 )
References:
WO2008/113760,2008,A2 Location in patent:Page/Page column 136