
ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate synthesis
- Product Name:ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate
- CAS Number:1059630-08-8
- Molecular formula:C14H17BrN2O2
- Molecular Weight:325.2
![(4aS,9bR)-6-bromo-1H,2H,3H,4H,4aH,5H,9bH-pyrido[4,3-b]indole](/CAS/20180713/GIF/1059630-13-5.gif)
1059630-13-5
51 suppliers
$45.00/50mg
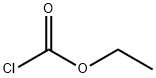
541-41-3
6 suppliers
$21.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
50 suppliers
$38.00/100mg
Yield:1059630-08-8 98%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran at 25; for 1.33333 h;Product distribution / selectivity;
Steps:
3
Example 3: Production of (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lH- pyrido[4,3-b]indoIe-2(9bH)-carboxylate; (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro- 1 H-pyrido[4,3-b]indole-2(9bH)-carboxylate may be prepared by first optaining [4aS, 9bR]-6-bromo- 2,3,4,4a,5,9b-hexahydro-lH-pyrido[4,3-Z>]indole (36.0 g, 0.142mol)) as a free base by using 50% aqueous sodium hydroxide solution and extracting the product into MTBE. The conversion to (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lη-pyrido[4,3- b]indole-2(9bH)-carboxylate may then be done by cooling a suspension of compounds of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-lH-pyrido[4,3-*]indole (36.0 g, 0.142mol)) in THF (300 ml) and triethylamine (24 ml) in an ice- water bath. Ethyl chloroformate is added dropwise (13.5 ml, 0.142mol) via a syringe pump over 1 hour. The ice-water bath is removed and the reaction mixture is stirred at room temperature for another hour. The reaction mixture is passed through a pad of celite and the solvent is evaporated to give (4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-lH-pyrido[4,3- b]indole-2(9bH)-carboxylate). 1H NMR (CDCl3, 300 MHz): 1.20-1.35 (m,3H), 1.73- 1.85 (m, IH), 1.85-1.99 (m, IH), 3.22-3.52 (m, 3H), 3.52-3.66 (m, IH), 3.66-3.95 (Br, IH), 3.95-4.21 (m, 4H), 6.60 (t, J = 7.7 Hz, IH), 7.04 (d, J = 7.2 Hz, IH), 7.20 (d, J = 8.1 Hz, IH).[0084] Alternative to the use of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro- lH-pyrido[4,3-Z>]indole (Compound of Formual 1C) free base, the reaction may also be done by starting with the (S)-mandelate salt of [4aS, 9bR]-6-bromo-2,3,4,4a,5,9b-
References:
WO2008/112280,2008,A1 Location in patent:Page/Page column 85-86
![(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole](/CAS/20200119/GIF/1059630-07-7.gif)
1059630-07-7
61 suppliers
inquiry

541-41-3
6 suppliers
$21.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
50 suppliers
$38.00/100mg
![1H-Pyrido[4,3-b]indole, 6-bromo-2,3,4,5-tetrahydro-, hydrochloride (1:1)](/CAS/20211123/GIF/1059630-11-3.gif)
1059630-11-3
36 suppliers
$90.00/50mg
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
50 suppliers
$38.00/100mg
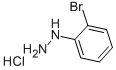
50709-33-6
284 suppliers
$5.00/1g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
50 suppliers
$38.00/100mg
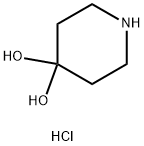
40064-34-4
366 suppliers
$10.00/5g
![ethyl(4aS,9bR)-6-bromo-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20180703/GIF/1059630-08-8.gif)
1059630-08-8
50 suppliers
$38.00/100mg