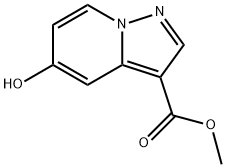
Methyl 5-hydroxypyrazolo[1,5-a]pyridine-3-carboxylate synthesis
- Product Name:Methyl 5-hydroxypyrazolo[1,5-a]pyridine-3-carboxylate
- CAS Number:1060724-74-4
- Molecular formula:C9H8N2O3
- Molecular Weight:192.17
![METHYL 5-(BENZYLOXY)PYRAZOLO[1,5-A]PYRIDINE-3-CARBOXYLATE](/CAS/GIF/866216-17-3.gif)
866216-17-3
15 suppliers
inquiry
![Methyl 5-hydroxypyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20180629/GIF/1060724-74-4.gif)
1060724-74-4
10 suppliers
inquiry
Yield:1060724-74-4 76%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20;
Steps:
1.c
Methyl 5-hydroxypyrazolo[1,5-a]pyridine-3-carboxylate (C4h); A suspension of 30 mg (0.11 mmol) methyl 5-benzyloxypyrazolo[1,5-a]pyridine-3-carboxylate (C5b) and 8 mg Pd/C (10%) in 5 ml ethanol were stirred over night under H2-atmosphere at room temperature. Then, the mixture was filtered over celite, evaporated and purified by flash-chromatography (hexane/EtOAc 1:1). Yield: 16 mg (76 %) white solid. Mp.: 264°C. MS (APCI): m/z 193 (M+1)+. IR (KBr) v (cm-1): 3435; 3057; 2949; 1699; 1647; 1541; 1277; 1248; 1045. 1H NMR (DMSO-d6, 600 MHz) δ (ppm): 3.79 (s, 3H, COOCH3); 6.70 (dd, J = 7.6 Hz, 2.6 Hz, 1H, H-6); 7.29 (d, J = 2.6 Hz, 1H, H-4); 8.27 (s, 1H, H-2); 8.67 (d, J = 7.6 Hz, 1H, H-7); 10.92 (s, 1H, OH). 13C NMR (DMSO-d6, 360 MHz) δ (ppm): 51.3; 99.2; 100.7; 108.7; 131.9; 143.0; 145.5; 158.8; 163.7.
References:
EP1972628,2008,A1 Location in patent:Page/Page column 32